Translate this page into:
Guidelines for the management of Stevens–Johnson syndrome/toxic epidermal necrolysis: An Indian perspective
2 Department of Dermatology, Baby Memorial Hospital, Kozhikode, Kerala, India
3 Department of Dermatology, Geetanjali Medical College, Udaipur, Rajasthan, India
4 Department of Dermatology, ESI-PGIMSR, New Delhi, India
5 Department of Dermatology, Medical College, Kolkata, West Bengal, India
6 Department of Dermatology, Grant Medical College, Mumbai, Maharashtra, India
7 Department of Dermatology, NKP Salve Institute of Medical Sciences, Nagpur, Maharashtra, India
8 Department of Dermatology, PSG Institute of Medical Sciences and Research, Coimbatore, Tamil Nadu, India
9 Department of Dermatology, Katihar Medical College, Katihar, Bihar, India
10 Department of Dermatology, Government Medical College, Gwalior, Madhya Pradesh, India
11 Department of Dermatology, All India Institute of Medical Sciences, Jodhpur, Rajasthan, India
Correspondence Address:
Lalit Kumar Gupta
Department of Dermatology, RNT Medical College, Udaipur, Rajasthan
India
| How to cite this article: Gupta LK, Martin AM, Agarwal N, D�Souza P, Das S, Kumar R, Pande S, Das NK, Kumaresan M, Kumar P, Garg A, Singh S. Guidelines for the management of Stevens–Johnson syndrome/toxic epidermal necrolysis: An Indian perspective. Indian J Dermatol Venereol Leprol 2016;82:603-625 |
Abstract
Background: Stevens–Johnson syndrome and toxic epidermal necrolysis are severe, life-threatening mucocutaneous adverse drug reactions with a high morbidity and mortality that require immediate medical care. The various immunomodulatory treatments include systemic corticosteroids, cyclosporine, intravenous immunoglobulin, cyclophosphamide, plasmapheresis and tumor necrosis factor-α inhibitors. Aim: The ideal therapy of Stevens–Johnson syndrome/toxic epidermal necrolysis still remains a matter of debate as there are only a limited number of studies of good quality comparing the usefulness of different specific treatments. The aim of this article is to comprehensively review the published medical literature and frame management guidelines suitable in the Indian perspective. Methods: The Indian Association of Dermatologists, Venereologists and Leprologists (IADVL) assigned the task of preparing these guidelines to its special interest group on cutaneous adverse drug reactions. The group performed a comprehensive English language literature search for management options in Stevens–Johnson syndrome/toxic epidermal necrolysis across multiple databases (PubMed, EMBASE, MEDLINE and Cochrane) for keywords (alone and in combination) and MeSH items such as “guidelines,” “Stevens–Johnson syndrome,” “toxic epidermal necrolysis,” “corticosteroids,” “intravenous immunoglobulin,” “cyclosporine” and “management.” The available evidence was evaluated using the strength of recommendation taxonomy and graded using a three-point scale. A draft of clinical recommendations was developed on the best available evidence which was also scrutinized and critically evaluated by the IADVL Academy of Dermatology. Based on the inputs received, this final consensus statement was prepared. Results: A total of 104 articles (meta-analyses, prospective and retrospective studies, reviews [including chapters in books], previous guidelines [including Indian guidelines of 2006] and case series) were critically evaluated and the evidence thus gathered was used in the preparation of these guidelines. Recommendations: This expert group recommends prompt withdrawal of the culprit drug, meticulous supportive care, and judicious and early (preferably within 72 h) initiation of moderate to high doses of oral or parenteral corticosteroids (prednisolone 1-2 mg/kg/day or equivalent), tapered rapidly within 7-10 days. Cyclosporine (3-5 mg/kg/day) for 10-14 days may also be used either alone, or in combination with corticosteroids. Owing to the systemic nature of the disease, a multidisciplinary approach in the management of these patients is helpful.Introduction
Background
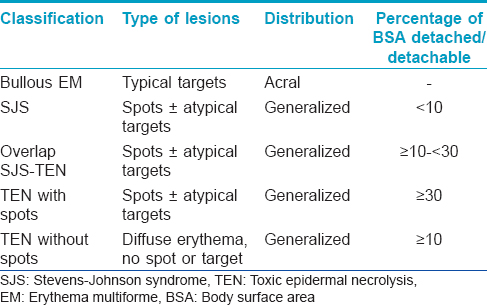
Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare, potentially life-threatening, severe mucocutaneous adverse reactions characterized by extensive epidermal detachment, erosion of mucosae and severe constitutional symptoms. Based on the similar histologic findings, SJS and TEN were synonymously associated with erythema multiforme major since 1983. However, Bastuji-Garin et al.[1] in 1993 and Roujeau [2] in 1994 proposed the differentiation of erythema multiforme from SJS and TEN based on clinical and etiologic information [Table - 1] and it remains the most acceptable classification till date. Stevens–Johnson syndrome, toxic epidermal necrolysis and overlap Stevens–Johnson syndrome/toxic epidermal necrolysis are considered to be variants within a continuous spectrum of epidermal necrolysis.
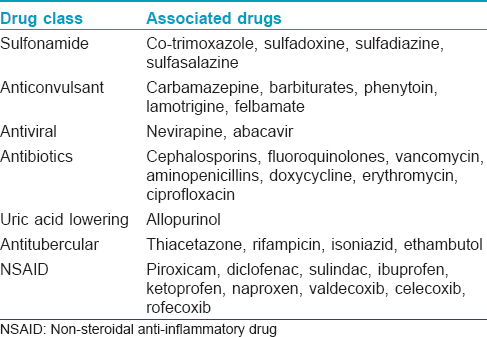
Most cases of SJS and TEN are drug-induced. Although any drug can cause SJS/TEN, the majority of reactions can be attributed to a group of high-risk drugs such as carbamazepine, phenytoin, allopurinol, lamotrigine, oxicam and other non-steroidal anti-inflammatory drugs, sulfonamide antibiotics and nevirapine [Table - 2].[3],[4] Mycoplasma pneumoniae and Cytomegalovirus infections are the next most common triggers of SJS/TEN, particularly in children.[5],[6]
People of specific ethnic groups with certain human leukocyte antigen subtypes have an increased incidence of SJS/TEN when exposed to specific drugs.[6],[7] An association between human leukocyte antigen-B*1502 and carbamazepine-induced Stevens-Johnson syndrome has been reported in Indian patients.[8] The usefulness of human leukocyte antigen screening in preventing SJS/TEN due to carbamazepine has been validated in Han Chinese patients with human leukocyte antigen-B*1502.[9] A systematic review and meta-analysis found a significant association between human leukocyte antigen-B*5801 and allopurinol-induced SJS/TEN in both Asian and non-Asian populations.[10] Patients with human immunodeficiency virus/acquired immune deficiency syndrome have a significantly greater risk of manifesting SJS/TEN.[11]
The present understanding of epidermal necrolysis is that it is an immune-driven pathway mediated by granulysin released by drug-specific cytotoxic CD8 T cells and natural killer cells.[12] Tumor necrosis factor-α, perforin/granzyme B, Fas ligand, Tweak and tumor necrosis factor-related apoptosis-inducing ligand which were earlier considered key mediators, are now thought to have a lesser, accessory role.[13]
Clinical features
The clinical features of SJS/TEN are characteristic and the diagnosis is primarily clinical.[14],[15] The initial symptoms or prodrome include fever, upper respiratory tract symptoms and conjunctivitis mimicking febrile illness of infective origin. This is followed by the detachment of mucous membranes (oropharyngeal, conjunctival, anogenital and nasal). Usually, more than two mucous membranes are involved. Cutaneous lesions, in the form of dusky erythematous macules/purpura and/or flat typical/atypical target lesions, erupt in association with pain and burning sensation. Typical raised target lesions, characteristic of the erythema multiforme spectrum, are usually absent. The lesions extend symmetrically, predominantly on the trunk and proximal limbs over a period of hours to 2–3 days. There is an appearance of flaccid blisters followed by sheet-like detachment of epidermis. Shearing pressure on the involved erythematous skin may cause epidermal detachment (pseudo-Nikolsky's sign). Peri-lesional erythema is a sign of disease activity and helps to monitor response to treatment.[16] Systemic symptoms are almost always associated with SJS/TEN overlap and toxic epidermal necrolysis. Involvement of the mucosae can lead to impaired alimentation, painful micturition, photophobia, diarrhea and respiratory distress. Thermoregulation is impaired and energy expenditure is increased. Re-epithelialization begins in a few days after the cessation of disease activity and is usually complete in about 3 weeks, barring mucosae and pressure sites which take longer.
Complications can cause both mortality and long-term morbidity in SJS/TEN. There is an increased risk of sepsis due to altered immune function. Dyspigmentation and scarring may develop. Ocular sequelae such as sicca syndrome, synechiae, scarring and blindness may develop. Hypopharyngeal stenosis combined with dysphagia, and esophageal strictures are long-term complications that are difficult to treat. Synechiae in other mucosae such as mouth and genitalia (esophagus or vaginal stenosis) may require surgery.
Differential diagnosis
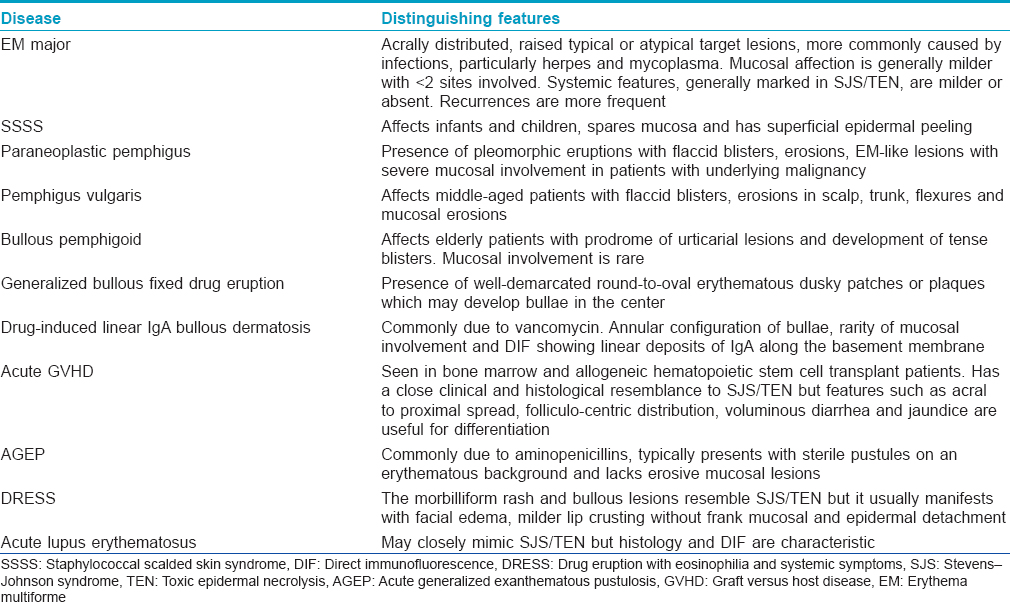
Although the clinical history and presentation of SJS/TEN is quite characteristic at a fully evolved stage, early lesions can mimic several bullous dermatoses and drug reactions [Table - 3].
Management
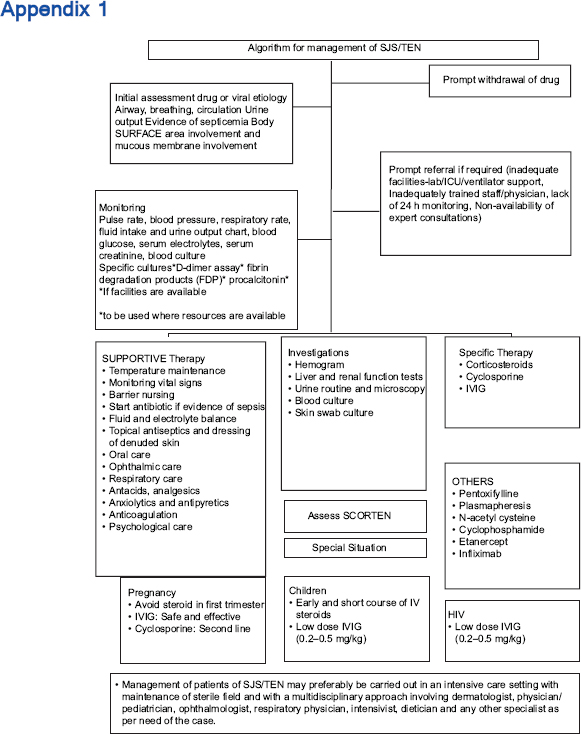
The management essentials include early recognition of the condition, cessation of suspected drug(s) if any, prompt institution of supportive therapy, referral if required, initiation of specific therapy, management of complications and prevention of future episodes. The various immunomodulatory treatments include systemic corticosteroids, cyclosporine, intravenous immunoglobulin, cyclophosphamide, plasmapheresis and tumor necrosis factor-α inhibitors. The ideal therapy still remains a matter of debate as there are only a limited number of studies of good quality comparing the usefulness of different specific treatments. The management and overall prognosis depend on the stage at which treatment is initiated, age of the patient, extent of necrolysis, associated comorbidities, accompanying complications (electrolyte imbalance, renal or hepatic dysfunction, adult respiratory distress syndrome and sepsis), the patient's ability to pay, drugs and resources available for patient care and the physician's experience with their use.
Prognosis
Prognostication is a complex exercise where the clinician has to take into account several parameters. Several factors may influence the outcome of SJS/TEN. Outcomes are based on several clinical and lab oratory parameters, age of the patient, co-morbidities, co-existing compromised disease states and physiologic states such as pregnancy. [Table - 4] lists the poor prognostic indicators in a clinical setting.
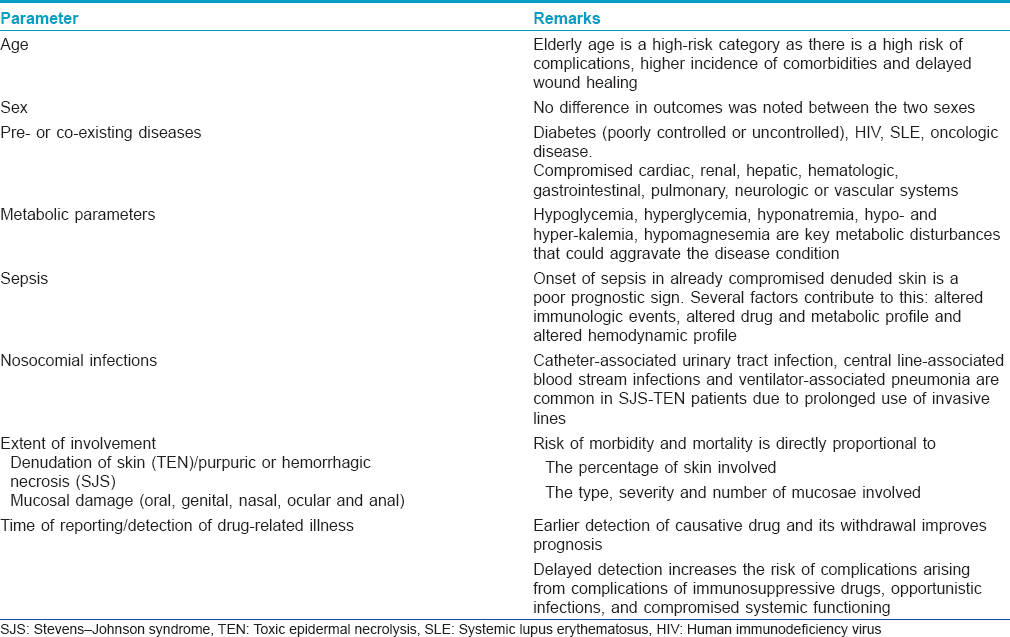
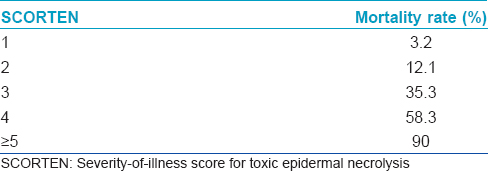
A validated mathematical tool named “severity-of-illness score for toxic epidermal necrolysis (SCORTEN)” for the prognostication of SJS/TEN patients has been developed.[17] It should be computed within 24 h after admission and again on the 3rd day. Some studies have suggested that the SCORTEN should be assessed on day 5. It is useful to predict the mortality and severity of illness. The index identifies seven independent risk factors for death [Box 1]. Each parameter is given a score of one and the total score is calculated by summing up the number of abnormal parameters. [Table - 5] shows the mortality rate according to the SCORTEN. These diseases are associated with a high morbidity and mortality; mortality rates are 1–5% with Stevens-Johnson syndrome, 10–15% with transitional forms and 25–30% with toxic epidermal necrolysis. The most common causes of death are sepsis, pulmonary failure and multiple organ failure.

Scope and Validity of These Guidelines
This document is a set of recommendations for evolving a standard of care in India for the management of patients with Stevens–Johnson syndrome/toxic epidermal necrolysis. They are intended for all clinicians involved in the care of suspected SJS/TEN patients at any level of health care: primary, secondary or tertiary. The purpose of these guidelines is to provide a framework for clinicians for effective, evidence-based management of patients of SJS/TEN, taking into consideration the minimum standard of care that may be provided in a resource-poor setting.
This consensus statement would be valid for 3 years from the date of publication when the document will be reviewed for validity based on the current best evidence at that time.
Methodology of Preparation of Guidelines
The special interest group on cutaneous adverse drug reactions (SIG-CADR) on behalf of the Indian Association of Dermatologists, Venereologists and Leprologists (IADVL) performed a comprehensive English language literature search for management options in SJS/TEN across multiple databases (PubMed, EMBASE, MEDLINE and Cochrane) for keywords (alone and in combination) and MeSH items such as “guidelines,” “Stevens–Johnson syndrome,” “toxic epidermal necrolysis,” “corticosteroids,” “intravenous immunoglobulin,” “IVIG,” “cyclosporine” and “management.” The available evidence was evaluated using a unified system called the strength of recommendation taxonomy (SORT) developed by editors of the US Family Medicine and Primary Care journals (i.e., American Family Physician, Family Medicine, Journal of Family Practice and British Medical Journal USA).[18]
Evidence was graded using a three-point scale based on the quality of methodology as follows:
- Good-quality patient-oriented evidence.
- Limited-quality patient-oriented evidence.
- Other evidence including consensus guidelines, opinion or case studies.
Clinical recommendations were developed on the best available evidence and ranked as follows:
- Recommendation based on consistent and good quality patient-oriented evidence.
- Recommendation based on inconsistent or limited-quality patient-oriented evidence.
- Recommendation based on consensus, opinion or case studies.
A draft was prepared which was then sent for review to an expert panel appointed by the IADVL Academy of Dermatology. It was also circulated among members of the e-groups of IADVL_Acad _IADVL for suggestions and criticism. Based on the inputs received, the final consensus statement was prepared.
Results
A total of 104 articles (meta-analyses, prospective and retrospective studies, reviews [including chapters in books], previous guidelines [including Indian guidelines of 2006 and UK guidelines of 2016] and case series) were critically evaluated and the evidence thus gathered was used in the preparation of these guidelines.
Management of patients essentially consists of:
- Prompt withdrawal of drug
- Initial assessment
- Prompt referral, if required
- Supportive treatment and investigations
- Initiation of disease-modifying therapy
- Prevention of recurrences.
Withdrawal of drug (level of evidence II, grade of recommendation B)
Instant cessation of the offending/suspected drug(s) is of supreme importance. Withdrawal of the offending drug will achieve the objective of aborting or slowing the process of acute skin failure and allow skin epithelialization in a shorter period of time. It has been shown that the earlier the causative drug is withdrawn, the better the prognosis and that patients exposed to causative drugs with long half-lives have an increased risk of dying.[19] It should be remembered that if the reaction has been caused by drugs with longer half-lives, the pathologic processes may continue for quite some time even after drug cessation. When the culprit drug is identified, it is easier to withdraw the offending drug. However, when multiple drugs (polypharmacy) or multi-drug combination therapies are used, it is difficult to ascertain the exact drug responsible for causing a drug reaction. Given the severity and life-threatening nature of SJS/TEN, it is generally logical to stop all drugs taken by the patient. However, if administration of a drug is absolutely essential (especially relevant for epileptics and also severe life-threatening infections such as septicemia), these drug(s) can be substituted with structurally unrelated drug(s). The decision to continue/substitute essential drugs should be taken in consultation with the treating clinician by following an interdisciplinary approach. It is important to restrict drug intake to the minimum possible.
Initial assessment
Patients with SJS/TEN should preferably be hospitalized. A detailed history must encompass the list of all drugs (including herbal, indigenous and other alternative system of medications) taken within a period of 2 months prior to the onset of the eruption. Co-morbidities (infections, immunosuppression, hepatic and renal disease, connective tissue disorders) and chronic diseases (such as diabetes mellitus and hypertension) should also be noted. The temporal association with the drug intake and previous episodes of allergic reactions are important parameters to incriminate a drug as an etiological agent. Personal or family history of drug reactions may be contributory and this must be recorded.
An initial assessment should include evaluation of airway, breathing and circulation, urinary output and any clinical evidence of septicemia. Percentage of body surface area involved and degree and the extent and number of mucosae involved need to be assessed and documented. The extent of erythema and the epidermal detachment, both detachable epidermis (i.e. Nikolsky-positive), as well as detached epidermis, should be recorded and depicted on a body map in the medical records. The same may also be documented with the help of photographs taken at the time of admission and repeated during the treatment at various stages. This helps to serve as an important safeguard against any potential medicolegal litigation.
Unfortunately, a confirmatory laboratory test that will allow the diagnosis of a drug reaction with some degree of certainty and identify the culprit drug is presently not available and those mentioned in the current literature are in experimental stages and have been used for research purposes. Hence, the recommended investigations for all the patients are for the purpose of knowing the degree of underlying damage to various organ systems, for prognosis and to plan further management [Appendix 1]. It should be noted that the severity of systemic damage may not necessarily correlate with that of epidermal necrolysis. The investigations include the following:
- Hemogram
- Liver and renal function tests
- Blood electrolytes
- Blood sugar
- Blood culture
- Urine routine and microscopy
- Skin swab culture
- Chest radiograph.
The patient and his/her relatives need to be taken into confidence and the severity, prognosis and potential for complications during the course of the disease need to be explained in language they understand.
Referral
Hospitalization in an intensive care setting or burns unit may be considered where such facilities are available. However, where there are limited resources, especially in rural or district hospitals, or where the patient may be unfit for transfer, they may need to be managed in the general/dermatology ward or high-dependency units with strict infection control with the help of nurses trained to deal with such cases. If possible, the patient should be admitted to an isolation room to facilitate infection and temperature control. Owing to the multisystem nature of disease, management should preferably be carried out by a team of experts including a dermatologist, physician, ophthalmologist, plastic surgeon, chest physician, dietician and any other specialists required as needed.
Supportive therapy (level of evidence II, grade of recommendation B)
Supportive treatment is essentially the same as for burn patients. For supportive treatment, this group recommends most of the guidelines as suggested by the previous IADVL Consensus Guidelines 2006: Management of SJS/TEN.[14] [Table - 6] enlists some of the important components of supportive care.
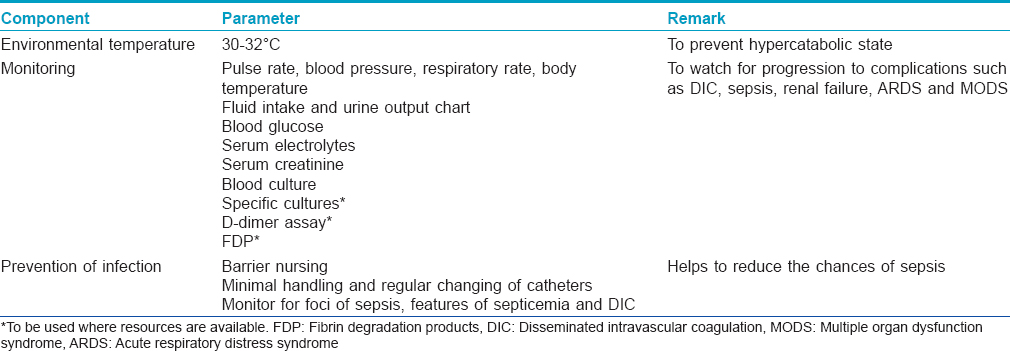
Environmental temperature maintenance
Environmental temperature maintenance at 30–32°C helps to prevent a hypercatabolic state by reducing caloric losses through the skin.[20] Heated-air body warmers may also be used.
Monitoring
Frequent monitoring of vital signs is an essential part of management as they offer the first sign of a worsening systemic condition. Monitoring (both initially at baseline and subsequently at periodic intervals) includes parameters such as pulse rate, blood pressure, respiratory rate, fluid intake and urine output chart, blood glucose, serum electrolytes, serum creatinine and specific cultures.
When the patient is on immunosuppressives, one must monitor for foci of sepsis (dental, gastrointestinal and urinary) that may flare up during the course of disease. Complications such as septicemia and disseminated intravascular coagulation can be monitored by specialized tests such as coagulation assays, D-dimer assay and fibrin degradation products, where facilities are available.
Preventing infection
The following measures are important to prevent sepsis in patients with SJS/TEN.
- Barrier nursing and sterile handling of the patient
- Regular hand hygiene with chlorhexidine hand rubs and hand washes to be practiced by health-care workers and caregivers
- Avoid unnecessary insertion of urinary catheters, intravenous lines or central lines
- If used, urinary catheters, intravenous lines and central lines must be handled minimally and changed regularly
- Monitor for foci of sepsis in the body, features of septicemia and disseminated intravascular coagulation
- Environmental controls for dependency units (air exchanges, humidity and temperature control) and intensive care unit
- Activate sepsis protocols early
- Judicious use of antibiotics.
In case barrier nursing is available, blood cultures are done at admission and then every 48 h. It is preferable to obtain the swab for culture and sensitivity from three lesional sites, particularly sloughed or crusted areas on alternate days throughout the acute phase.[21] Antibiotics can be initiated either when direct proof of sepsis exists, i.e., when blood cultures are positive or as soon as indirect signs of sepsis occur, i.e., general deterioration in the form of hypothermia, fever or shivering after the 4th day, diminishing level of consciousness, confused mental status, anxiety/excitement, falling urine output, deterioration of respiratory status/diabetic control, failure of gastric emptying and any sudden change in the condition.[22] Staphylococcus aureus is the main bacterium present during the first few days; Gram-negative strains appear later. It should be kept in mind that systemic steroids may mask the signs of sepsis.
Since most centers do not have the infrastructure for barrier nursing, prophylactic antibiotic therapy may be considered for widespread skin involvement and slightest clinical suspicion of sepsis. Empirical coverage should include one antibiotic each having anti-staphylococcal activity (amoxicillin + clavulanic acid/tetracyclines/vancomycin/clindamycin/teicoplanin/linezolid), Gram-negative activity (amikacin/piperacillin + tazobactam/cefoperazone + sulbactam/imipenem) and anaerobic activity (metronidazole/tinidazole). If there is even slight suspicion that SJS/TEN has been caused by a particular antibiotic(s), it is important to strictly avoid that antibiotic group and use an alternative, structurally unrelated agent. [Box 2] lists the definite indications of antibiotic use in SJS/TEN patients.[22]

Fluid and electrolyte balance
- Peripheral intravenous line can be left in place for a long duration and should not be replaced before 96 h unless there is evidence of phlebitis, local infection or malfunction. Routine culture of vascular catheter tip is not recommended.[23]
- Patients of toxic epidermal necrolysis lose a significant amount of fluid as blister fluid and insensible fluid loss and should be assumed to be hypovolemic. Adult patients having inolvement of 50% of body surface area lose around 3–4 L of fluid every day. This is usually accompanied by the loss of electrolytes such as sodium, potassium and chloride in blister fluid. Hypophosphatemia is a common complication in these patients aggravating insulin resistance and altering the neurological status and diaphragmatic functions.[22] If replacement is not given promptly, the patient may develop dehydration which may adversely affect the outcome. Urine becomes hyperosmolar and urine output decreases. Slowly, the serum urea and creatinine concentration increases and pre-renal failure may develop.
- The early fluid requirement of TEN patients is two-third to three-fourth of that of a burn patient with the same extent of skin involvement and should be fulfilled by macromolecules (Ringer lactate) or saline solutions.[24] The fluid requirements of burn patients is calculated by the Parkland formula, as follows:
Fluid requirement = 4 ml/kg body weight × percentage of body surface area involved.
- For the first 24 h, half the calculated fluid is administered in the first 8 h and the other half in the next 16 h. Fluid requirements beyond the first 24 h should be managed according to the patient's condition. Input and output charting is useful to guide fluid administration. The maintenance fluid is titrated so as to maintain a urine output between 1000 and 1500 ml. It must be noted that overcorrection of hypovolemia may also lead to pulmonary edema. Blood transfusion may be useful in some cases and perhaps works by the dilution of drug metabolites, cytokines, cytotoxic T-cells and autoantibodies, providing immunoglobulins and nutrition, besides correcting anemia and hypovolemia.[25]
Feeding
After admission, an oral liquid diet, nasogastric tube or total parenteral nutrition should be initiated. If feasible, oral feeding is always preferred though it is often difficult because of upper gastrointestinal tract injury. In the early stages of disease when dysphagia and odynophagia are severe, a fluid/semisolid diet is preferable and better tolerated by the patient. Early and continuous enteral nutrition decreases the risk of stress ulcers, reduces bacterial translocation and enterogenic infection and allows early discontinuation of venous lines. Caloric requirements are calculated as 30–35 kcal/kg/day. Proteins (approximately 1.5 g/kg/day) are given to avoid a negative nitrogen balance.
Topical antiseptics and dressing of denuded skin
It is advisable to leave detached/detachable epidermis in place to provide a natural dressing. Regular cleaning of wounds with clean water or normal saline is important, whether the patient is bedridden or mobile, to remove the infected crusts as this reduces the chances of infection. Gentian violet paint in dilution and silver nitrate (0.5%) are useful for treating denuded areas. Silver sulfadiazine should be avoided because of frequent implication of sulphonamides in SJS/TEN.[14] Dressing is useful to prevent heat losses, infection, adhesion of clothes to raw skin surfaces and to promote re-epithelialization. For denuded areas, dressing can be done with paraffin or petrolatum gauze, with or without antibiotic impregnation. Adhesive dressings should be avoided. Frequent changes in patient position and an air-fluid bed/water bed help in early healing of lesions, prevention of bed sores and reduce patient discomfort. Burn cage is also useful in preventing adhesion of denuded areas to clothes in cases of extensive involvement.
Oral and ophthalmic care
Oral hygiene should be maintained with normal saline swishes or antiseptic or anesthetic mouth washes. Saline compresses followed by the application of lubricants can be advised for the lips. This also helps soften hemorrhagic lip crusts. Daily examination by an ophthalmologist and vigorous treatment reduce the risk of long-term ocular complications. Lubrication and antibiotic eye drops/ointments with or without corticosteroids are needed frequently (every 2 h). Lid globe adhesions should be cautiously removed with a glass rod daily. Recently, the application of amniotic membranes was reported to be effective in preserving visual acuity and an intact ocular surface.[26]
Respiratory care
Lung involvement may be complicated by pulmonary edema during fluid replacement. Pulmonary care includes normal saline aerosols, bronchial aspiration and postural drainage by turning the patient to different sides. Pooling of saliva and secretions may predispose to aspiration and therefore need to be cleared frequently. Hypostatic pneumonia should be prevented by frequent change of posture and mobilization of the patient as early as possible. The nose may require attention in the form of moisturization with saline and removal of adherent crusts.
Antacids, analgesics, anxiolytics and antipyretics
Antacids (H2 receptor antagonists and proton pump inhibitors) reduce the incidence of gastric bleeding. Pain management has to be individualized taking into consideration the clinical needs of the patient, viable route of administration, risk of respiratory suppression and monitoring facilities. Opioids (such as tramadol) and anxiolytics may be helpful. Core body temperature >39°C should prompt measures to cool the patient (cooled intravenous fluids and cold sponges). Nonsteroidal anti-inflammatory drugs such as antipyretics/analgesics must not be used if suspected to be the causative agent of SJS/TEN.
Anticoagulation
Thromboembolism and disseminated intravascular coagulation are important causes of morbidity and death and hence, anticoagulation with low-molecular weight heparin may be useful in some patients.[19] Early mobilization of the patient may also be helpful.
Psychological care
Providing emotional support and maintaining a continual dialogue with the patient and his/her family is a vital part of supportive care and addresses the patient's fears/anxieties, improves compliance with daily nursing care and gives an opportunity for patient education about self-care after discharge and prevention of future episodes.
Investigations
The role of investigations in SJS/TEN is primarily for the detection of systemic involvement, prognostication and guiding therapy rather than diagnosis, which is essentially clinical. There is no reliable and validated in vitro or in vivo diagnostic test for SJS/TEN. The laboratory changes commonly include anemia and lymphopenia; neutropenia is seen in one-third of the patients and is associated with a poor prognosis. Hyperglycemia and glycosuria may occur due to stress, infection and possibly pancreatitis and indicate a poor prognosis. Liver enzymes are elevated in half of the patients and occasionally, frank hepatitis may develop, induced by drugs, sepsis or shock. Hematuria and proteinuria may be seen indicating renal involvement. Blood urea nitrogen and serum creatinine levels may be raised because of dehydration. Serum electrolyte levels may guide fluid and electrolyte administration. Chest radiograph, blood and skin swab culture, human immunodeficiency virus testing (enzyme-linked immunosorbent assay) and skin biopsy may be done if indicated.
The interest in investigations has now shifted to detect the early markers of SJS/TEN. Soluble Fas ligand, perforin/granzyme B, soluble CD40 ligand and granulysin have been studied with granulysin emerging to be the most useful of these markers. A rapid immunochromatographic assay capable of detecting high levels of serum granulysin (>10 ng/ml) has been developed with a sensitivity of 80% and a specificity of 95.8% in detecting SJS/TEN when compared to ordinary cutaneous drug reactions.[13] The other emerging markers for the early detection of SJS/TEN are serum lactate dehydrogenase, thymus and activation-regulated chemokine (TARC/CCL17), glutathione-S-transferase-pi (GSTpi) and high-mobility group protein B1 (HMGB1), alpha-defensins 1–3 in blister fluid and Bcl-2 expression in dermal infiltrate.[13]
Disease-modifying therapy
Disease-modifying therapy in SJS/TEN is aimed at halting the immunological processes leading to keratinocyte apoptosis. Various immunomodulating agents such as systemic corticosteroids, cyclosporine, intravenous immunoglobulin, plasmapheresis, tumor necrosis factor-α inhibitors, granulocyte colony-stimulating factor and N-acetylcysteine have been used with variable results across the globe.
Systemic corticosteroids (level of evidence II, grade of recommendation B)
Traditionally, systemic corticosteroids have remained the mainstay of therapy of Stevens-Johnson syndrome and toxic epidermal necrolysis in most centers. The rationale is that both these consitions are immune-mediated processes and corticosteroids suppress the intensity of the reaction, prevent/decrease the necrolysis of skin, reduce fever and discomfort and prevent damage to internal organs when given at an early stage and in sufficiently high dosage.[27],[28],[29],[30],[31],[32],[33]
Evidence for the use of steroids
The largest study to evaluate the effect of treatments, including steroids, was performed by Schneck et al.[34] The authors examined data from a case–control study that evaluated the risk factors for Stevens-Johnson syndrome or toxic epidermal necrolysis in six countries in Europe in a cohort of 379 patients with confirmed disease. The authors concluded that there was inadequate evidence that any specific treatment is established as effective with only corticosteroids showing a trend for possible benefit. They called for “a prospective randomized trial to be conducted before any conclusions can be drawn, recommending that corticosteroids be trialled first.” A recently published review identified six retrospective studies which analyzed the role of steroids in the survival of patients with SJS/TEN.[34],[35],[36],[37],[38],[39],[40] They concluded that “a review of the highest quality evidence available for steroid use in patients withStevens-Johnson syndrome, SJS/ TEN overlap and toxic epidermal necrolysis does not reveal an increase in mortality.”There have been several other international reports supporting the role of corticosteroids in patients with SJS/TEN.[26],[41],[42],[43],[44],[45],[46],[47],[48],[49]
Among Indian studies, Pasricha et al., in multiple reports, strongly recommended the use of high-dose short-duration corticosteroids in the routine management of SJS/TEN.16, 50, 51 The same has been emphasized in other Indian publications.[52],[53],[54],[55]
Ocular sequelae are one of the major complications of Stevens-Johnson syndrome and toxic epidermal necrolysis. A few reports support the use of corticosteroids in preventing ocular sequelae also.[26],[41] These studies also emphasize that the early use of corticosteroids halts disease progression and consequently complications.
When should steroids be given?
Most authors agree that corticosteroids are useful when given at an early stage.41-47,52-56 Dhar has very aptly stated that “it is the lack of knowledge and expertise about how to use it rather than the lack of its efficacy which has made the role of corticosteroid(s) a matter of debate.”[52]Das et al. opined that systemic corticosteroids are effective in controlling the disease process as well as recovery if started preferably within 3 days (maximum up to 7 days) from the onset of toxic epidermal necrolysis in resource-poor settings.[54] They also stated that systemic corticosteroids in the initial phase of disease continue to be life-saving drugs in developing countries such as ours. Kardaun and Jonkman concurred that the “general negative opinion of corticosteroids is probably because they are often given too late, in too low a dose and for too long a period” and admitted that during “the healing phase, corticosteroids may indeed impair wound healing and promote sepsis.”[46] They also challenged the general opinion that systemic corticosteroids are detrimental in Stevens-Johnson syndrome and toxic epidermal necrolysis and felt that “short courses of high-dose corticosteroids in early SJS/TEN have a good rationale, as immune mechanisms are directly responsible for the cascade of events leading to apoptosis.” A review by Kakourou et al. concluded that an early and short course of systemic corticosteroid therapy provides a favorable influence on the outcome.[47]
How much and by what route?
The dosing, duration and route of administration that is most effective in SJS/TEN patients is open to debate. Tapering of corticosteroids over 7–10 days to as long as 4 weeks has also been reported.[43],[57]
High/suprapharmacological doses of corticosteroids as conventional therapy or pulse or a combination of both have been advocated.41, 46, 48, 49, 54 Das et al. administered dexamethasone at a dose of 1 mg/kg to 18 patients with toxic epidermal necrolysis who had had the eruption for 7 days or less. Corticosteroids were tapered and withdrawn within 5 days after the subsidence of erythema and there was no mortality in the group.[54] Intravenous pulsed dose methyl prednisolone (3 consecutive daily infusions of 20–30 mg/kg to a maximum of 500 mg given over 2–3 h) has also been successfully given.[49] In addition, Hirahara et al. gave an infusion of methylprednisolone at 1000 mg/day for 3 consecutive days, followed by oral prednisolone at 0.8–1 mg/kg/day in tapering doses in eight patients with no mortality.[48] Kardaun and Jonkman have recently proposed dexamethasone pulse therapy (1.5 mg/kg intravenous over 30–60 min on 3 consecutive days) to avoid long-term use of systemic corticosteroids.[46] The authors described the pleomorphic effects of dexamethasone on the immune system including the inhibition of epidermal apoptosis by several mechanisms. These mechanisms include the suppression of various cytokines, such as tumor necrosis factor-α, interferon-γ, interlekuin-6 and interlekuin-10; inhibition of interferon-γ-induced apoptosis and inhibition of Fas-mediated keratinocyte apoptosis. Rai and Srinivas have successfully used suprapharmacological doses of dexamethasone along with cyclosporine in their patients.[55]
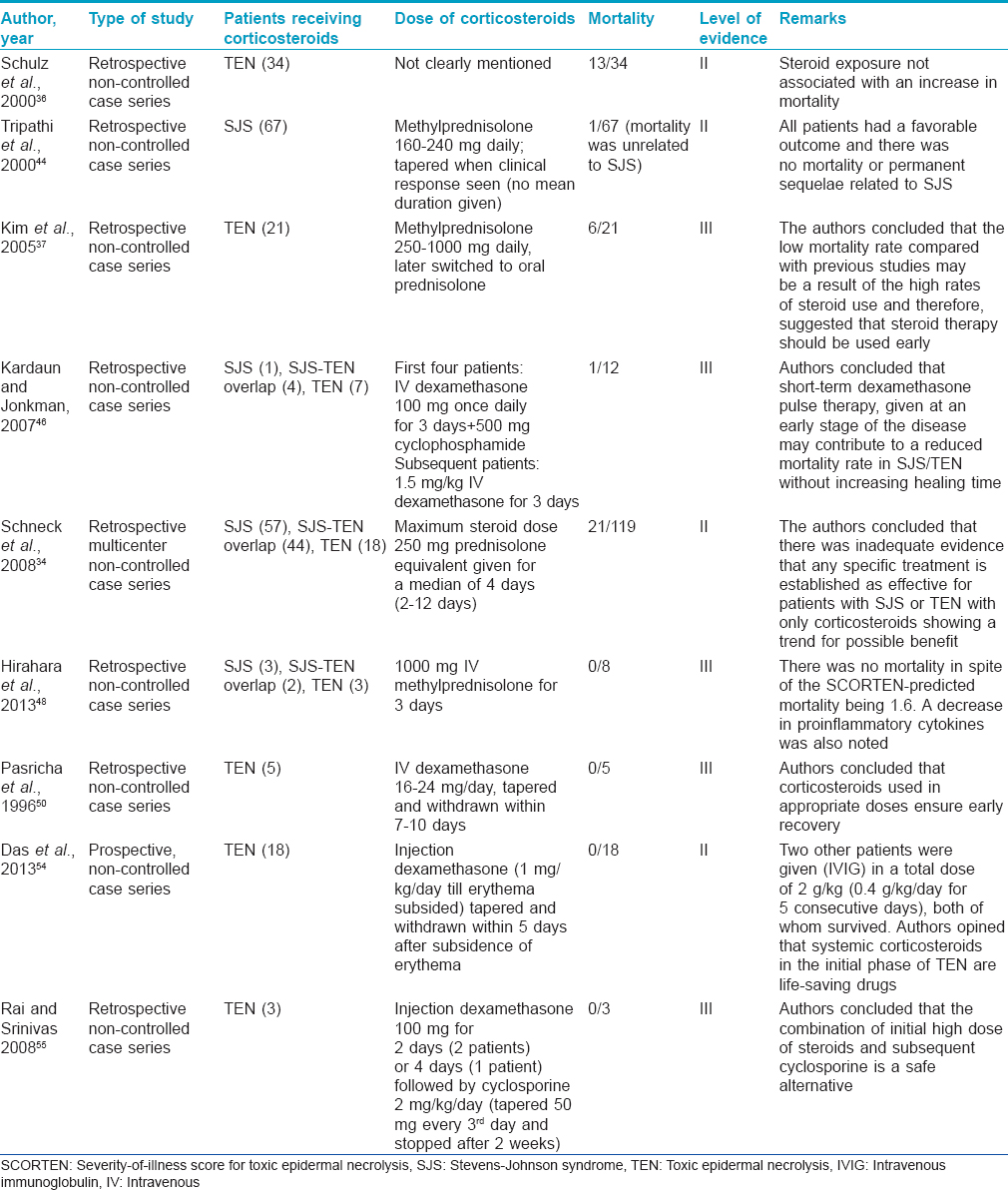
Advocates of systemic corticosteroids recommend the administration of a high dose (regardless of route) early in the course of the disease for a short period of time. [Table - 7] summarizes the various studies on corticosteroid usage in SJS/TEN.
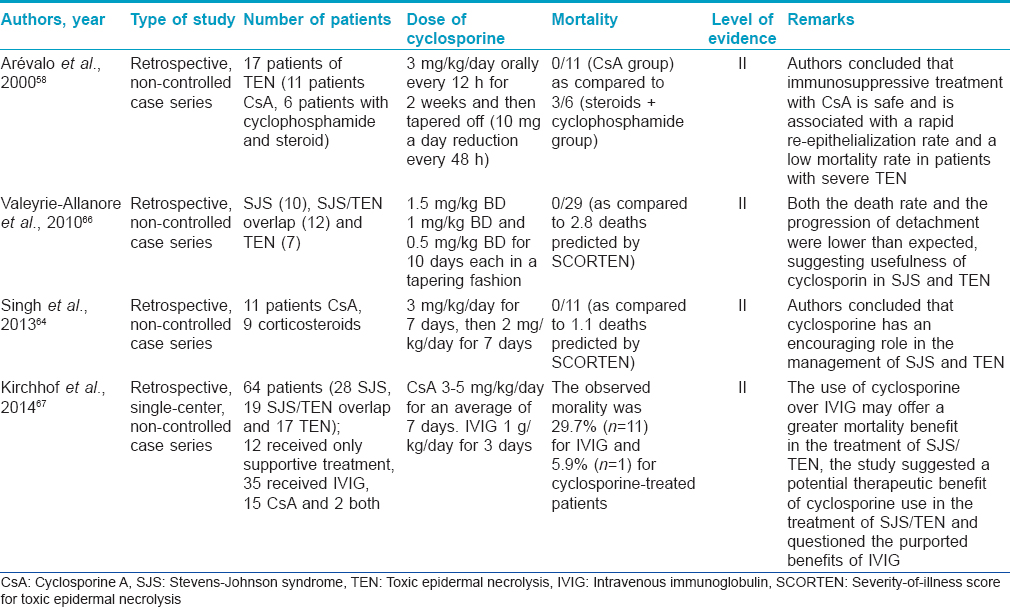
Cyclosporine (level of evidence II, grade of recommendation B)
In recent years, cyclosporine has gained popularity in the treatment of SJS/TEN. The use of cyclosporine in SJS/TEN was suggested based on the role of T-lymphocytes in the pathogenesis of toxic epidermal necrolysis and striking clinical and histologic similarity of the disease to some cases of acute graft versus host disease. Cyclosporine (CsA) inhibits the activation of CD4+ and CD8+ (cytotoxic) T-cells in the epidermis by suppressing interlekuin-2 production from activated T helper cells. Many case reports, case series, open trials and retrospective studies have documented the efficacy of cyclosporine in SJS/TEN.[58],[59],[60],[61],[62],[63],[64],[65],[66] In fact, some of these reports suggest the superiorty of cyclosporine over other therapies including intravenous immunoglobulin, corticosteroids, cyclophosphamide and supportive care alone.
Cyclosporine is used in a dose of 3–5 mg/kg body weight, as oral capsules or solution, in divided doses. However, there is apparently no consensus on the duration of therapy. Most authors have used it for 1 month, or till resolution of skin lesions and re-epithelization. It has been suggested that long-term treatment is probably unnecessary as the disease progression generally stops before the 10th day of cyclosporine administration. Hence, it appears that short duration treatment (7–10 days) is equally useful and cost-effective, an important issue in a developing country like India.
In an Indian study, 11 patients treated with cyclosporine at a dose of 3 mg/kg/day for 7 days (and then tapered for another 7 days) were retrospectively compared with nine patients treated with corticosteroids. Cyclosporine significantly reduced the time to the arrest of progression of SJS/TEN, the total re-epithelialization time and hospital stay in comparison to corticosteroids.[64] Cyclosporine in combination with suprapharmacological doses of intravenous dexamethasone has been successfully used in the treatment of SJS/TEN.[55] In another Indian report, cyclosporine was used to successfully treat toxic epidermal necrolysis induced by cyclophosphamide.[65]
In a recent comparative study between cyclosporine and intravenous immunoglobulin in SJS/TEN, the expected mortality rate based on SCORTEN in 17 patients treated with cyclosporine was 14.1%; however, the observed mortality rate was 5.9%.[67] Similar figures for 37 patients treated with intravenous immunoglobulin were 20.8% (expected mortality rate) and 29.7% (observed mortality rate). The calculated standardized mortality ratio also suggested a survival benefit to cyclosporine use.
Most common side effects associated with long-term cyclosporin treatment such as hypertension and renal toxicity are not seen in treatment with short duration of treatment. However, septic complications and severe leukopenia (<1000 cells/mm [3]) should be watched out for.
[Table - 8] summarizes the various studies on cyclosporine usage in SJS/TEN.
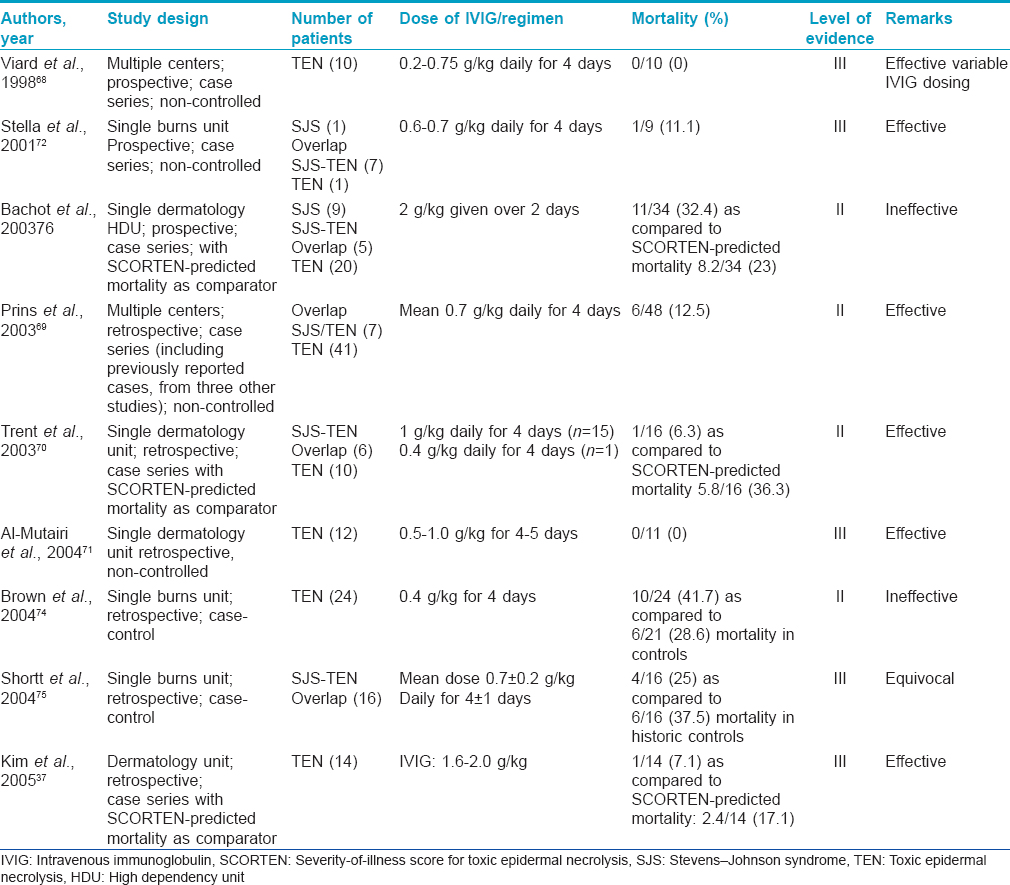
Intravenous immunoglobulin (level of evidence II, grade of recommendation B)
A perception that use of corticosteroids leads to enhanced mortality in SJS/TEN led to the search for alternative immunomodulatory agent(s). In 1998, Viard et al. demonstrated the reversal of Fas-mediated keratinocyte apoptosis by human immunoglobulin by in vitro studies.[68] Since then, many studies have investigated the role of intravenous immunoglobulin, but the results have been conflicting. Several studies have reported a decreased mortality in patients with TEN treated with intravenous immunoglobulin. These studies found survival rates of 88%, 94% and 100% with total intravenous immunoglobulin doses of 2.7, 4 and 3.4 g/kg, respectively.69-73 On the contrary, studies have also found no mortality benefit compared with supportive therapy alone or when compared with the mortality predicted by SCORTEN.34,74-76 The largest study to date (EuroSCAR) on the treatment of SJS/TEN found that 75 patients treated with an average total dose of 1.5–1.9 g/kg of intravenous immunoglobulin did not have improved mortality compared with the group that received supportive therapy alone.[34] More recently, skepticism surrounding the role of intravenous immunoglobulin has surfaced. A retrospective study of 64 patients treated with intravenous immunoglobulin concluded that the use of intravenous immunoglobulin in the treatment of SJS/TEN overlap and toxic epidermal necrolysis does not yield survival benefits, even when corrected for intravenous immunoglobulin dosages, i.e., low dose (<3 g/kg) or high dose (>3 g/kg) and prior exposure to corticosteroids.[77] The only meta-analysis of intravenous immunoglobulin studies in SJS/TEN has been done by Huang et al. which indicated uncertainty regarding the efficacy of intravenous immunoglobulin in TEN.[78] They found that the intravenous immunoglobulin dose, when adjusted for age, total body surface area involvement and delay of treatment, did not correlate with mortality benefits. This becomes more convincing in the light of evidence negating the role of Fas-Fas ligand-mediated necrosis of epidermal cells and the emergence of the role of granulysin as the primary pathogenetic mediator.[12]
There have been a few Indian studies on intravenous immunoglobulin, used either alone or in combination, in SJS/TEN.54, 73, 79 Two of these studies used extremely low doses of intravenous immunoglobulin (cumulative dose of <0.5 g/kg) and had favorable results. This might be a more feasible option in resource-poor countries such as ours where the prohibitively high cost of intravenous immunoglobulin therapy is an important constraint. It has been suggested that the addition of corticosteroids to low-dose intravenous immunoglobulin might increase efficacy by a possible synergistic effect.[79]
As outlined above, there has been a significant lack of consistency in the efficacy of intravenous immunoglobulin in different reports. Any decisive conclusion is complicated by a lack of homogeneity in many key clinical areas such as disease severity, patient comorbidities, dose, duration and timing of intravenous immunoglobulin administration. The inconsistencies can also be attributed to the variability in the potential to inhibit Fas-mediated cell death among intravenous immunoglobulin batches. Knowledge of the possible adverse effects/complications of intravenous immunoglobulin therapy is essential before the initiation of treatment. These include a risk of anaphylaxis in patients with IgA deficiency and that of acute renal failure in patients with cryoglobulinemia. Therapy with high-dose intravenous immunoglobulin should be used cautiously in patients with renal insufficiency or impaired cardiac function because fluid overload may occur. [Table - 9] summarizes the various studies on intravenous immunoglobulin usage in SJS/TEN.
Other therapies (level of evidence III, grade of recommendation C)
Plasmapheresis, pentoxifylline, N-acetyl cysteine and cyclophosphamide have been reported to have some benefit in cases with SJS/TEN,80-86 but other studies have not found any definite benefit.[87],[88] Granulocyte colony-stimulating factor was previously given as an adjuvant therapy in SJS/TEN patients with severe neutropenia, but of late, its efficacy in patients without neutropenia has also been suggested.89-91
A trial involving tumor necrosis factor-α inhibitor thalidomide was prematurely suspended due to higher mortality in the thalidomide-treated group and hence it is not recommended in SJS/TEN.[92] However, subsequent case reports have shown favorable outcomes in patients treated with anti-tumor necrosis factor-α biologicals such as infliximab and etanercept.93-95
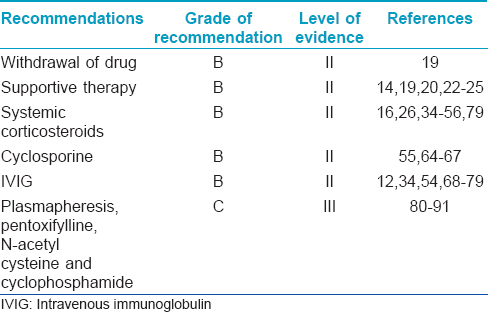
The various aspects of the management of SJS/TEN with their evidence levels and grade of recommendation are summarized in [Table - 10].
Prevention of recurrences, follow-up and counseling
Preventing the recurrence of reaction is an important aspect of management in any form of drug reaction, particularly in severe types like SJS/TEN. The episode should be reported to the national pharmacovigilance authorities. Drug allergy should be documented in the patient's case sheet and the discharge ticket very legibly, preferably in red ink. The patient or his/her attendant should be given written information about drug(s) to avoid. They should be issued a drug reaction card mentioning the suspected drug(s) and encouraged to carry this card in their pocket all the time and show it to the clinician every time they fall ill. The patient should also be advised to seek appropriate consultations for the management of complications/sequelae resulting from SJS/TEN, particularly ophthalmological complications. SJS/TEN patients and their first-degree relatives have been known to exhibit a defect in the detoxification of reactive metabolites and hence, survivors and their first-degree relatives should avoid suspected offending agents and related compounds. For example, a patient developing SJS/TEN to carbamazepine should avoid phenytoin and phenobarbital which can be substituted with unrelated compounds such as sodium valproate or lamotrigine. In addition, β-lactam antibiotics, cephalosporins and carbapenems should be strictly avoided in any patient with a history of SJS/TEN to penicillin or any other β-lactam antibiotic. Drugs of the same pharmacologic class can be used, provided they are structurally different from the culprit drug. For example, arylamine containing sulphonamides such as sulfamethoxazole, sulfadiazine, sulfapyridine and sulfamethizole cross react, whereas sulphonamide diuretics, hypoglycemics, sulfasalazine and sulfisoxazole are unrelated compounds which can be given in these patients. Patients should be issued a drug card to be carried at all times and asked to produce it at the time of visiting any health-care professional. Provocation tests are not advisable routinely in SJS/TEN; however, it is an established protocol in some centers such as the All India Institute of Medical Sciences, New Delhi. It helps patients, especially with multiple comorbidities having alleged history of drug reaction to multiple drugs. These patients greatly suffer due to unwillingness and apprehension on the part of physicians to prescribe them appropriate medications for the fear of precipitating drug rash. In such cases, providing these patients a safe drug list by undertaking supervised drug administration may be highly gratifying.
In centers where provocation tests are not routinely undertaken, an algorithm for drug causality for epidermal necrolysis (ALDEN) developed by Sassolas et al. can be useful for ascertaining drug causality.[96] ALDEN assigns each drug a score from -12 to +10 based on six parameters: (1) the time delay from initial drug intake to onset of reaction; (2) the probability of drug presence in the body on the index day; (3) a previous history of adverse reaction to the same drug; (4) the presence of the drug beyond the progression phase of the disease; (5) the drug notoriety based on the previous results of the EuroSCAR study [4] and (6) the presence or absence of other etiologic causes. The score is categorized as very probable (>6); probable (4–5); possible (2–3); unlikely (0–1) and very unlikely (0).
Stevens–Johnson syndrome/toxic epidermal necrolysis in pregnancy
Toxic epidermal necrolysis in pregnancy puts two lives at risk and hence, it requires the immediate attention of both dermatologist and gynecologist. A large majority of patients who develop SJS/TEN in pregnancy are human immunodeficiency virus-positive and nevirapine is the most common drug incriminated.[97]
Effect on mother
Pregnancy as such is not a risk factor for mortality in toxic epidermal necrolysis.[90] A review of the literature by Struck et al. supports a high survival rate of both mother and child.[98] Long-term complications of genital involvement in SJS/TEN include vaginal stenosis and adhesions, endometriosis and telangiectasia.[99] To prevent vaginal complications, a vaginal mold with local corticosteroids/lubricant gel can be inserted in the vagina to prevent adhesions.[100]
Effect on fetus
Maternal toxic epidermal necrolysis is known to cause fetal stress and preterm labor.[98] Hence, it is prudent to keep a close watch on fetal parameters during management. In most cases, the child is not affected by toxic epidermal necrolysis.[97] In fact, toxic epidermal necrolysis in neonates/early infancy is very rare and only some cases have been reported. Rodriguez et al. have reported a case of toxic epidermal necrolysis in both a mother and her stillborn child.[101]
Management
Management of toxic epidermal necrolysis in pregnant women is not very different from that of non-pregnant patients. Systemic steroids should not be favored in the first trimester, but may be useful in the third trimester as they help to increase the lung maturity of fetus, more so in case of preterm labor. Intravenous immunoglobulin and cyclosporine (pregnancy category C) can also be used in individual cases.[102] Intravenous immunoglobulin has been used safely in treating pregnant patients with toxic epidermal necrolysis.[101],[103] There is not much literature on the use of cyclosporine in pregnant women with toxic epidermal necrolysis. The use of cyclosporine in pregnant renal transplant recipients has been associated with adverse effects in newborns.[104] However, these adverse effects are usually seen with long-term use of cyclosporine to prevent rejection and toxic epidermal necrolysis does not require long-term treatment.
Stevens–Johnson syndrome/toxic epidermal necrolysis in children
Stevens–Johnson syndrome/toxic epidermal necrolysis in children does not differ significantly in its etiology, clinical features and management strategies from adult SJS/TEN. Some points, however, are worth mentioning. Drugs are the most common culprits in SJS/TEN (both children and adults). However, the likelihood of infections (Mycoplasma and Cytomegalovirus) inducing SJS/TEN is relatively higher in children as compared to adults.[5],[6] Sulphonamides, penicillins and nonsteroidal anti-inflammatory drugs are more commonly implicated in drug-induced pediatric SJS/TEN owing to their more frequent use in children.[105] Anticonvulsants are another class of drugs commonly implicated in SJS/TEN in children. Stevens–Johnson syndrome and toxic epidermal necrolysis rarely cause mortality in children, but significant morbidity is seen.[105] In a study of 21 patients by Prendiville et al., there was an excellent response to supportive care alone which included reverse barrier isolation, intravenous fluids and nutritional support, meticulous skin care, early detection and treatment of infection and daily ophthalmologic examination, with no mortality.[30] It has been seen that the energy needs of children with SJS/TEN are 22% less than the age and wound size matched burn patients. The recommended equation for the estimation of energy requirements in children with SJS/TEN is: (24.6 × weight in kg) + (% wound × 4.1) + 940.[106]
Corticosteroids, intravenous immunoglobulin and cyclosporine have been utilized for specific therapy of pediatric SJS/TEN in various studies. Kakourou et al. suggest a bolus infusion of methylprednisolone (4 mg/kg/day) for 3–7 days which showed a significant reduction of the period of fever and acute eruption and milder signs of prostration as compared to supportive therapy alone.[47] No relapses occurred after treatment was discontinued. They concluded that an early and short course of intravenous corticosteroids favorably influenced the course of SJS/TEN in children. Intravenous immunoglobulin has been used as a treatment with mixed results. An Indian study by Mangla et al. evaluated the role of low-dose intravenous immunoglobulin (0.05–0.1 mg/kg/day for 5 days) in pediatric toxic epidermal necrolysis and found it to be a safe and effective treatment.[74] Cyclosporine has also shown promising results in the treatment of pediatric SJS and TEN.[107]
Discussion
Some general questions relevant to a physician managing SJS/TEN are as follows:
Should disease-modifying therapy be administered or not?
Due to ethical reasons, there is insufficient evidence comparing supportive therapy alone versus supportive therapy combined with disease-modifying agents. Based on vast personal experience and clinical observations by the members of this group, members of the expert review committee, academicians and senior practitioners across India, it is recommended that systemic disease-modifying therapy be used early in the disease to help prevent progression of the disease.
What is an ideal disease-modifying therapy?
The special interest group on cutaneous adverse drug reactions (SIG–CADR) acknowledges the lacunae in knowledge in medical literature (both Indian and international). Lack of head-to-head, comparative, clinical, double-blinded trials to identify the efficacy and safety of immunosuppressive drugs further makes it difficult to decide on the best therapy. No single immunosuppressive drug can be considered universally efficacious and superior to others in arresting the immunologic damage caused by the disease. Thus, the issue still remains unresolved. However, a thorough scrutiny of the pros and cons of different disease-modifying drugs has been done later in the text to arrive at a consensus.
What is the dilemma regarding the use of corticosteroids in Stevens–Johnson syndrome/toxic epidermal necrolysis?
Prior to the 1970s, systemic corticosteroids were used as a standard therapy for patients with Stevens-Johnson syndrome. A report by Rasmussen et al. presented the initial data challenging the role of systemic corticosteroids in treating Stevens-Johnson syndrome, pointing to longer hospital stays and higher complication rates in children who received this treatment.[108] In the 1980s, in Western countries, the management of SJS and TEN shifted to specialized burn units and was taken over by non-dermatologists, mostly plastic surgeons. The important role of intensive care units in the management of SJS and TEN notwithstanding, it brought about a paradigm shift in the way these diseases were perceived. Surgeons outrightly rejected the use of steroids and regarded them as the cause of increased morbidity and complications (e.g., sepsis, leukopenia, thromboembolism and gastrointestinal ulcerations), prolonged recovery, worse prognosis and reduced survival. Following widely cited reports, there was a complete turnaround in the management, to a non-steroidal approach.109-114 These developments brought skepticism in the usefulness of corticosteroids in SJS/TEN among dermatologists who adopted defensive medicine. Fine, however, carefully scrutinized this literature and revealed many methodological problems.56,108-114 The report by Rasmussen did not specify the doses of corticosteroids used.[108] One widely cited paper reported higher mortality in patients treated with high-dose corticosteroids as compared to those which were not.[109] However, 73% of patients in the group described as not having been treated with corticosteroids (with higher survival rate) had in fact received corticosteroids for a mean of 3.5 days which may have contributed to their higher survival rate. Furthermore, patients with toxic epidermal necrolysis were intermixed with patients with Stevens-Johnson syndrome and it was not clear whether the two entities were distributed equally in each treatment group, even though their mortality rates differ greatly. In addition, too few details about the corticosteroid therapy were provided to permit any critical assessment of the treatment. This was the case in other studies also, in which corticosteroid duration and dose had not been specified.[108],[115] These are crucial flaws because corticosteroid therapy given for too short a time, at too low a dose or too late in the disease course may not have been of much benefit. Another interesting point has been made by Wolf and Davidovici that plastic surgeons in-charge of burn care units view burn and toxic epidermal necrolysis as similar entities and although there is an acute skin failure in both conditions, they differ significantly in terms of etiology and pathomechanism.[116] Burn is a one-time acute event which affects the skin from outside whereas toxic epidermal necrolysis is a more complex immune-mediated process that reaches the skin from within. Above all, unlike thermal burns, toxic epidermal necrolysis continues to progress and becomes more intensified over a period of days. Therefore, it would make sense to turn to aggressive disease-modifying drugs that are capable of halting disease progression, reduce the extent of skin detachment and decrease inflammatory cytokines, organ damage and mortality. In addition, many of these studies from burn centers had some common authors and possibility of an investigator bias cannot be excluded.109-112
When should disease-modifying therapy be started?
The optimum time for starting disease-modifying therapy has not been sufficiently scrutinized in the medical literature. However, most experts advocate starting them early and tapering down quickly to counter the risks involved with immunosuppressive therapy.
The benefits of starting such medications early in the disease include the following:
- The progression of immunologic cellular damage is prevented
- The extent of denudation of skin is limited
- Relief of pain, burning, fever and pruritus
- Systemic complications (lung, renal, cardiac, hepatic, nervous and gastrointestinal) due to immunologic damage are curtailed
- Morbidity due to scarring and strictures is limited.
What if the patient reports late or the diagnosis is delayed
No consensus could be reached on whether systemic immunosuppressives can be used when the drug reaction has been detected late (more than 7 days duration). Some members were of the view that immunosuppressive therapy can be life-saving in such situations too, if sufficient monitoring mechanisms, nursing care requirements and appropriate antibiotic coverage can be provided in an advanced care setting.
The special interest group on cutaneous adverse drug reactions (SIG-CADR) of the IADVL is of the view that therapy needs to be individualized taking into consideration the following:
- The stage at which the treatment is initiated
- Age of the patient
- Extent of necrolysis
- Associated comorbidities
- Accompanying complications (electrolyte imbalance, renal or hepatic dysfunction, adult respiratory distress syndrome and sepsis)
- Patient's ability to afford interventions, drugs and resources available for patient care and the physician's experience with their use.
Summary of the Indian Guidelines and Recommendations
For the management of Stevens-Johnson syndrome, SJS/TEN and toxic epidermal necrolysis, the Indian Association of Dermatologists, Venereologists and Leprologists's special interest group on cutaneous adverse drug reactions (SIG-CADR) recommends:
- Immediate withdrawal of all suspected/offending drug(s) and related compounds (grade of recommendation B).
- Initiation of supportive therapy as the primary measure to be undertaken in all patients of SJS/TEN presenting to a health-care professional (grade of recommendation B).
- If the rash has been identified at a primary or secondary health-care center, the treatment should be initialized and thereafter referred to a tertiary care center for care by a dermatologist.
- If resources are available, the treatment may be carried out in an intensive care setting or in an isolated room with maintenance of sterile field. A multidisciplinary approach involving dermatologist, physician/pediatrician, ophthalmologist, respiratory physician, intensivist, dietician and any other specialist as per the need of the case should be adopted.
- Disease-modifying treatment must be initiated as early as possible.
- Systemic corticosteroids (preferably parenteral) are recommended as the disease-modifying treatment of choice (grade of recommendation B). Prednisolone, dexamethasone or methylprednisolone should be given early (preferably within 72 h) in high dosage (1–2 mg/kg/day prednisolone or 8–16 mg/day of dexamethasone intravenous or intramuscular). A daily assessment of disease activity (such as the appearance of new lesions, peri-lesional erythema and skin tenderness) should be done and steroids should be maintained at the same dose till disease activity ceases. Thereafter, dosage should be tapered quickly such that the total duration of steroid therapy is around 7–10 days. Steroids can also be administered in pulse form employing slow intravenous infusion of methylprednisolone (500–1000 mg/day) or dexamethasone (100 mg) for 3 days.
- Cyclosporine (grade of recommendation B) can also be employed alone (3–5 mg/kg/day for 10–14 days), especially in patients with relative contraindications to corticosteroid use (e.g., patients with tuberculosis and severe hyperglycemia).
- If both steroids and cyclosporine are used, steroids can be tapered even more quickly (2–3 days) and cyclosporine (3–5 mg/kg/day) can be continued for 7–10 days.
- If a patient reports at a stage when the disease activity has already ceased, there is no need of any disease-modifying treatment. Such patients should be managed by supportive therapy alone.
- Monitoring and management of complications (vital signs, signs of sepsis and systemic involvement) and sequelae with the help of a multidisciplinary team of specialists is important.
- In patients with human immunodeficiency virus, children and pregnant women in the first trimester, low-dose of intravenous immunoglobulin (cumulative dose 0.2–0.5 mg/kg) may be considered (grade of recommendation B), given in the first 24–48 h.
- Strict avoidance of offending/suspected/related drug(s) is absolutely necessary. A drug card should be issued to facilitate this.
Disclaimer/legal standing of recommendations
- The“Indian Association of Dermatologists, Venereologists and Leprologists- special interest group-cutaneous adverse drug reactionsrecommendations”is the consensus view of the current working group and does not hold the validity of a legal document in a court of law or stand legal scrutiny. However, this statement may be admissible as “Recommendations for reasonable standard of care in the management of SJS/TEN patients in India.” The members of the group cannot be held responsible for legal consequences that may arise from following the recommendations mentioned in this document
- Adhering to these recommendations does not ensure perfect outcomes or ensure complete cure in all patients. The measures mentioned are an aid to correctly diagnose, investigate, foresee and manage complications and treat appropriately
- The group realizes that SJS and TEN are rare and extremely complex diseases to manage and treat. The recommendations by this group do not overrun the appropriateness of decisions taken by the treating physician
- This document will serve as a guide to assess and treat patients in a wise and informed manner, based on evidence from the current medical literature and the experience of several physicians across the country.
Acknowledgments
We appreciate the guidance received from Dr. Venkataram Mysore first as the Therapeutic Guidelines Committee Chairman 2012 and subsequently as the president of IADVL (2015), for his personal interest, academic inputs and coordination. We also thank the other members such as Dr. Rajesh M. Buddhadev, Dr. Satish Pai, Dr. Vinay Saraf and Dr. Krupashankar of the Therapeutic Guidelines Committee.
We gratefully acknowledge the chairperson and convener of IADVL Academy Dr. Manas Chatterjee and Dr. Ameet Valia, and Hon' secretary of IADVL Dr. Rashmi Sarkar for entrusting the responsibility of framing these guidelines to the special interest group on cutaneous adverse drug reactions.
A large portion of “supportive care” in SJS/TEN in the draft has been adopted from the previously published guidelines on the management of SJS/TEN by IADVL'sTherapeutic Guidelines Committee 2006. We sincerely thank all the members of the committee including Dr. V. K. Sharma, Dr. H. R. Jerajani, Dr. C. R. Srinivas, Dr. Ameet Valia and Dr. Sujay Khandpur.
We sincerely thank the members of Expert Review Committee appointed by IADVL: Dr. C. R. Srinivas, Dr. Murlidhar Rajgopalan, Dr. Sunil Dogra, Dr. V. K. Sharma and Dr. Sandipan Dhar who critically reviewed the document and provided their useful suggestions.
Several members including Dr. M. Ramam, Dr. Manas Chatterjee, Dr. Suresh Talwar, Dr. Kiran Godse, Dr. Abir Saraswat, Dr. Abhishek De, Dr. Nitin Walia, Dr. Rakesh Bharti, Dr. Manish Pahwa, Dr. Kingshuk Chatterjee, Dr. R. Manjunath, Dr. Vikas Yatagiri, Dr. Vinay Kulkarni, Dr. Bela Shah, Dr. Asit Mittal and Dr. A. K. Khare provided their valuable inputs on the documents through the IADVL_Acad group and we immensely thank all of them.
We deeply acknowledge the assistance of Dr. M. Ramam, Dr. Riti Bhatia, Dr. Akhilesh Shukla, Dr. Ranjana Beniwal, Dr. Ankita Srivastav and Dr. Chesta Agarwal who helped in proofreading and editing the manuscript on several occasions.
| 1. |
Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 1993;129:92-6.
[Google Scholar]
|
| 2. |
Roujeau JC. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: A clinical classification. J Invest Dermatol 1994;102:28S-30S.
[Google Scholar]
|
| 3. |
Mockenhaupt M. The current understanding of Stevens-Johnson syndrome and toxic epidermal necrolysis. Expert Rev Clin Immunol 2011;7:803-13.
[Google Scholar]
|
| 4. |
Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: Assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol 2008;128:35-44.
[Google Scholar]
|
| 5. |
Wetter DA, Camilleri MJ. Clinical, etiologic, and histopathologic features of Stevens-Johnson syndrome during an 8-year period at Mayo Clinic. Mayo Clin Proc 2010;85:131-8.
[Google Scholar]
|
| 6. |
Ferrandiz-Pulido C, Garcia-Patos V. A review of causes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Arch Dis Child 2013;98:998-1003.
[Google Scholar]
|
| 7. |
Dao RL, Su SC, Chung WH. Recent advances of pharmacogenomics in severe cutaneous adverse reactions: Immune and nonimmune mechanisms. Asia Pac Allergy 2015;5:59-67.
[Google Scholar]
|
| 8. |
Mehta TY, Prajapati LM, Mittal B, Joshi CG, Sheth JJ, Patel DB, et al. Association of HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian J Dermatol Venereol Leprol 2009;75:579-82.
[Google Scholar]
|
| 9. |
Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF, Yang CC, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med 2011;364:1126-33.
[Google Scholar]
|
| 10. |
Somkrua R, Eickman EE, Saokaew S, Lohitnavy M, Chaiyakunapruk N. Association of HLA-B*5801 allele and allopurinol-induced Stevens Johnson syndrome and toxic epidermal necrolysis: A systematic review and meta-analysis. BMC Med Genet 2011;12:118.
[Google Scholar]
|
| 11. |
Mittmann N, Knowles SR, Koo M, Shear NH, Rachlis A, Rourke SB. Incidence of toxic epidermal necrolysis and Stevens-Johnson syndrome in an HIV cohort: An observational, retrospective case series study. Am J Clin Dermatol 2012;13:49-54.
[Google Scholar]
|
| 12. |
Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med 2008;14:1343-50.
[Google Scholar]
|
| 13. |
Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol 2013;69:187.e1-16.
[Google Scholar]
|
| 14. |
Sharma VK, Jerajani HR, Srinivas CR, Valia A, Khandpur S. IADVL Consensus Guidelines 2006: Management of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. In: Sharma VK, editor. Guidelines for vitiligo, Stevens-Johnson syndrome, toxic epidermal necrolysis and psoriasis. 2nd ed. New Delhi: IADVL's Therapeutic Guidelines Committee; 2008.
[Google Scholar]
|
| 15. |
Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol 2013;69:173.e1-13.
[Google Scholar]
|
| 16. |
Pasricha JS. Management of toxic epidermal necrolysis. Indian J Dermatol Venereol Leprol 1990;50:458-9.
[Google Scholar]
|
| 17. |
Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: A severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol 2000;115:149-53.
[Google Scholar]
|
| 18. |
Ebell MH, Siwek J, Weiss BD, Woolf SH, Susman JL, Ewigman B, et al. Simplifying the language of evidence to improve patient care: Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in medical literature. J Fam Pract 2004;53:111-20.
[Google Scholar]
|
| 19. |
Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson syndrome: Does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol 2000;136:323-7.
[Google Scholar]
|
| 20. |
Revuz J, Roujeau JC, Guillaume JC, Penso D, Touraine R. Treatment of toxic epidermal necrolysis. Créteil's experience. Arch Dermatol 1987;123:1156-8.
[Google Scholar]
|
| 21. |
Creamer D, Walsh SA, Dziewulski P, Exton LS, Lee HY, Dart JK, et al. UK guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. J Plast Reconstr Aesthet Surg 2016;69:e119-53.
[Google Scholar]
|
| 22. |
Inamadar AC, Palit A. Acute skin failure: Concept, causes, consequences and care. Indian J Dermatol Venereol Leprol 2005;71:379-85.
[Google Scholar]
|
| 23. |
Committee for the Development of Guidelines for the Prevention of Vascular Catheter Associated Infection; Indian Society of Critical Care Medicine. Epidemiology. Indian J Crit Care Med 2003;7, Suppl S1:6.
[Google Scholar]
|
| 24. |
Roujeau JC, Revuz J. Intensive care in dermatology. In: Champion RH, Pye RJ, editors. Recent Advances in Dermatology. Edinburg: Churchill Livingstone; 1990. p. 85-99.
[Google Scholar]
|
| 25. |
Dhar S. Role of blood transfusion in the management of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). Indian J Dermatol Venereol Leprol 1998;64:250-1.
[Google Scholar]
|
| 26. |
Kim KH, Park SW, Kim MK, Wee WR. Effect of age and early intervention with a systemic steroid, intravenous immunoglobulin or amniotic membrane transplantation on the ocular outcomes of patients with Stevens-Johnson syndrome. Korean J Ophthalmol 2013;27:331-40.
[Google Scholar]
|
| 27. |
Björnberg A. Fifteen cases of toxic epidermal necrolysis (Lyell). Acta Derm Venereol 1973;53:149-52.
[Google Scholar]
|
| 28. |
Ohlenschlaeger K. Toxic epidermal necrolysis and Stevens-Johnson's disease. Acta Derm Venereol 1966;46:204-9.
[Google Scholar]
|
| 29. |
Sherertz EF, Jegasothy BV, Lazarus GS. Phenytoin hypersensitivity reaction presenting with toxic epidermal necrolysis and severe hepatitis. Report of a patient treated with corticosteroid “pulse therapy”. J Am Acad Dermatol 1985;12(1 Pt 2):178-81.
[Google Scholar]
|
| 30. |
Prendiville JS, Hebert AA, Greenwald MJ, Esterly NB. Management of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. J Pediatr 1989;115:881-7.
[Google Scholar]
|
| 31. |
Tegelberg-Stassen MJ, van Vloten WA, Baart de la Faille H. Management of nonstaphylococcal toxic epidermal necrolysis: Follow-up study of 16 case histories. Dermatologica 1990;180:124-9.
[Google Scholar]
|
| 32. |
Patterson R, Grammer LC, Greenberger PA, Lawrence ID, Zeiss CR, Detjen PF, et al. Stevens-Johnson syndrome (SJS): Effectiveness of corticosteroids in management and recurrent SJS. Allergy Proc 1992;13:89-95.
[Google Scholar]
|
| 33. |
Cheriyan S, Patterson R, Greenberger PA, Grammer LC, Latall J. The outcome of Stevens-Johnson syndrome treated with corticosteroids. Allergy Proc 1995;16:151-5.
[Google Scholar]
|
| 34. |
Schneck J, Fagot JP, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: A retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol 2008;58:33-40.
[Google Scholar]
|
| 35. |
Law EH, Leung M. Corticosteroids in Stevens-Johnson syndrome/toxic epidermal necrolysis: Current evidence and implications for future research. Ann Pharmacother 2015;49:335-42.
[Google Scholar]
|
| 36. |
Schulz JT, Sheridan RL, Ryan CM, MacKool B, Tompkins RG. A 10-year experience with toxic epidermal necrolysis. J Burn Care Rehabil 2000;21:199-204.
[Google Scholar]
|
| 37. |
Kim KJ, Lee DP, Suh HS, Lee MW, Choi JH, Moon KC, et al. Toxic epidermal necrolysis: Analysis of clinical course and SCORTEN-based comparison of mortality rate and treatment modalities in Korean patients. Acta Derm Venereol 2005;85:497-502.
[Google Scholar]
|
| 38. |
Yang Y, Xu J, Li F, Zhu X. Combination therapy of intravenous immunoglobulin and corticosteroid in the treatment of toxic epidermal necrolysis and Stevens-Johnson syndrome: A retrospective comparative study in China. Int J Dermatol 2009;48:1122-8.
[Google Scholar]
|
| 39. |
Kim HI, Kim SW, Park GY, Kwon EG, Kim HH, Jeong JY, et al. Causes and treatment outcomes of Stevens-Johnson syndrome and toxic epidermal necrolysis in 82 adult patients. Korean J Intern Med 2012;27:203-10.
[Google Scholar]
|
| 40. |
Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol 2013;133:1197-204.
[Google Scholar]
|
| 41. |
Araki Y, Sotozono C, Inatomi T, Ueta M, Yokoi N, Ueda E, et al. Successful treatment of Stevens-Johnson syndrome with steroid pulse therapy at disease onset. Am J Ophthalmol 2009;147:1004-11, 1011.e1.
[Google Scholar]
|
| 42. |
Marvin JA, Heimbach DM, Engrav LH, Harnar TJ. Improved treatment of the Stevens-Johnson syndrome. Arch Surg 1984;119:601-5.
[Google Scholar]
|
| 43. |
Patterson R, Miller M, Kaplan M, Doan T, Brown J, Detjen P, et al. Effectiveness of early therapy with corticosteroids in Stevens-Johnson syndrome: Experience with 41 cases and a hypothesis regarding pathogenesis. Ann Allergy 1994;73:27-34.
[Google Scholar]
|
| 44. |
Tripathi A, Ditto AM, Grammer LC, Greenberger PA, McGrath KG, Zeiss CR, et al. Corticosteroid therapy in an additional 13 cases of Stevens-Johnson syndrome: A total series of 67 cases. Allergy Asthma Proc 2000;21:101-5.
[Google Scholar]
|
| 45. |
Su P, Aw CW. Severe cutaneous adverse reactions in a local hospital setting: A 5-year retrospective study. Int J Dermatol 2014;53:1339-45.
[Google Scholar]
|
| 46. |
Kardaun SH, Jonkman MF. Dexamethasone pulse therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol 2007;87:144-8.
[Google Scholar]
|
| 47. |
Kakourou T, Klontza D, Soteropoulou F, Kattamis C. Corticosteroid treatment of erythema multiforme major (Stevens-Johnson syndrome) in children. Eur J Pediatr 1997;156:90-3.
[Google Scholar]
|
| 48. |
Hirahara K, Kano Y, Sato Y, Horie C, Okazaki A, Ishida T, et al. Methylprednisolone pulse therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis: Clinical evaluation and analysis of biomarkers. J Am Acad Dermatol 2013;69:496-8.
[Google Scholar]
|
| 49. |
Martinez AE, Atherton DJ. High-dose systemic corticosteroids can arrest recurrences of severe mucocutaneous erythema multiforme. Pediatr Dermatol 2000;17:87-90.
[Google Scholar]
|
| 50. |
Pasricha JS, Khaitan BK, Shantharaman R, Mital A, Girdhar M. Toxic epidermal necrolysis. Int J Dermatol 1996;35:523-7.
[Google Scholar]
|
| 51. |
Pasricha JS. Corticosteroids in toxic epidermal necrolysis. Indian J Dermatol Venereol Leprol 2008;74:493.
[Google Scholar]
|
| 52. |
Dhar S. Systemic corticosteroids in toxic epidermal necrolysis. Indian J Dermatol Venereol Leprol 1996;62:270-1.
[Google Scholar]
|
| 53. |
Pasricha JS. Corticosteroids in toxic epidermal necrolysis. Indian J Dermatol Venereol Leprol 2008;74:493.
[Google Scholar]
|
| 54. |
Das S, Roy AK, Biswas I. A six month prospective study to find out the treatment outcome, prognosis and offending drugs in toxic epidermal necrolysis from an urban institution in Kolkata. Indian J Dermatol 2013;58:191-3.
[Google Scholar]
|
| 55. |
Rai R, Srinivas CR. Suprapharmacologic doses of intravenous dexamethasone followed by cyclosporine in the treatment of toxic epidermal necrolysis. Indian J Dermatol Venereol Leprol 2008;74:263-5.
[Google Scholar]
|
| 56. |
Fine JD. Management of acquired bullous skin diseases. N Engl J Med 1995;333:1475-84.
[Google Scholar]
|
| 57. |
Chrousos GP. Adrenocorticosteroids and adrenocortical antagonists. In: Katzung BG, editor. Basic and Clinical Pharmacology. 9th ed. New York: Lange Medical Books/McGraw-Hill; 2004. p. 641-60.
[Google Scholar]
|
| 58. |
Arévalo JM, Lorente JA, González-Herrada C, Jiménez-Reyes J. Treatment of toxic epidermal necrolysis with cyclosporin A. J Trauma 2000;48:473-8.
[Google Scholar]
|
| 59. |
Aihara Y, Ito R, Ito S, Aihara M, Yokota S. Toxic epidermal necrolysis in a child successfully treated with cyclosporin A and methylprednisolone. Pediatr Int 2007;49:659-62.
[Google Scholar]
|
| 60. |
Hashim N, Bandara D, Tan E, Ilchyshyn A. Early cyclosporine treatment of incipient toxic epidermal necrolysis induced by concomitant use of lamotrigine and sodium valproate. Acta Derm Venereol 2004;84:90-1.
[Google Scholar]
|
| 61. |
Hewitt J, Ormerod AD. Toxic epidermal necrolysis treated with cyclosporin. Clin Exp Dermatol 1992;17:264-5.
[Google Scholar]
|
| 62. |
Reese D, Henning JS, Rockers K, Ladd D, Gilson R. Cyclosporine for SJS/TEN: A case series and review of the literature. Cutis 2011;87:24-9.
[Google Scholar]
|
| 63. |
Renfro L, Grant-Kels JM, Daman LA. Drug-induced toxic epidermal necrolysis treated with cyclosporin. Int J Dermatol 1989;28:441-4.
[Google Scholar]
|
| 64. |
Singh GK, Chatterjee M, Verma R. Cyclosporine in Stevens Johnson syndrome and toxic epidermal necrolysis and retrospective comparison with systemic corticosteroid. Indian J Dermatol Venereol Leprol 2013;79:686-92.
[Google Scholar]
|
| 65. |
Patel MP, Kute VB, Vanikar AV, Trivedi HL. Cyclophosphamide-induced toxic epidermal necrolysis: Vigilance needed. Clin Kidney J 2014;7:323-4.
[Google Scholar]
|
| 66. |
Valeyrie-Allanore L, Wolkenstein P, Brochard L, Ortonne N, Maître B, Revuz J, et al. Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol 2010;163:847-53.
[Google Scholar]
|
| 67. |
Kirchhof MG, Miliszewski MA, Sikora S, Papp A, Dutz JP. Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol 2014;71:941-7.
[Google Scholar]
|
| 68. |
Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science 1998;282:490-3.
[Google Scholar]
|
| 69. |
Prins C, Kerdel FA, Padilla RS, Hunziker T, Chimenti S, Viard I, et al. Treatment of toxic epidermal necrolysis with high-dose intravenous immunoglobulins: Multicenter retrospective analysis of 48 consecutive cases. Arch Dermatol 2003;139:26-32.
[Google Scholar]
|
| 70. |
Trent JT, Kirsner RS, Romanelli P, Kerdel FA. Analysis of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis using SCORTEN: The University of Miami Experience. Arch Dermatol 2003;139:39-43.
[Google Scholar]
|
| 71. |
Al-Mutairi N, Arun J, Osama NE, Amr Z, Mazen AS, Ibtesam el-A, et al. Prospective, noncomparative open study from Kuwait of the role of intravenous immunoglobulin in the treatment of toxic epidermal necrolysis. Int J Dermatol 2004;43:847-51.
[Google Scholar]
|
| 72. |
Stella M, Cassano P, Bollero D, Clemente A, Giorio G. Toxic epidermal necrolysis treated with intravenous high-dose immunoglobulins: Our experience. Dermatology 2001;203:45-9.
[Google Scholar]
|
| 73. |
Mangla K, Rastogi S, Goyal P, Solanki RB, Rawal RC. Efficacy of low dose intravenous immunoglobulins in children with toxic epidermal necrolysis: An open uncontrolled study. Indian J Dermatol Venereol Leprol 2005;71:398-400.
[Google Scholar]
|
| 74. |
Brown KM, Silver GM, Halerz M, Walaszek P, Sandroni A, Gamelli RL. Toxic epidermal necrolysis: Does immunoglobulin make a difference? J Burn Care Rehabil 2004;25:81-8.
[Google Scholar]
|
| 75. |
Shortt R, Gomez M, Mittman N, Cartotto R. Intravenous immunoglobulin does not improve outcome in toxic epidermal necrolysis. J Burn Care Rehabil 2004;25:246-55.
[Google Scholar]
|
| 76. |
Bachot N, Revuz J, Roujeau JC. Intravenous immunoglobulin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis: A prospective noncomparative study showing no benefit on mortality or progression. Arch Dermatol 2003;139:33-6.
[Google Scholar]
|
| 77. |
Lee HY, Lim YL, Thirumoorthy T, Pang SM. The role of intravenous immunoglobulin in toxic epidermal necrolysis: A retrospective analysis of 64 patients managed in a specialized centre. Br J Dermatol 2013;169:1304-9.
[Google Scholar]
|
| 78. |
Huang YC, Li YC, Chen TJ. The efficacy of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: A systematic review and meta-analysis. Br J Dermatol 2012;167:424-32.
[Google Scholar]
|
| 79. |
Jagadeesan S, Sobhanakumari K, Sadanandan SM, Ravindran S, Divakaran MV, Skaria L, et al. Low dose intravenous immunoglobulins and steroids in toxic epidermal necrolysis: A prospective comparative open-labelled study of 36 cases. Indian J Dermatol Venereol Leprol 2013;79:506-11.
[Google Scholar]
|
| 80. |
Narita YM, Hirahara K, Mizukawa Y, Kano Y, Shiohara T. Efficacy of plasmapheresis for the treatment of severe toxic epidermal necrolysis: Is cytokine expression analysis useful in predicting its therapeutic efficacy? J Dermatol 2011;38:236-45.
[Google Scholar]
|
| 81. |
Sullivan JR, Shear NH. The drug hypersensitivity syndrome: What is the pathogenesis? Arch Dermatol 2001;137:357-64.
[Google Scholar]
|
| 82. |
Kamanabroo D, Schmitz-Landgraf W, Czarnetzki BM. Plasmapheresis in severe drug-induced toxic epidermal necrolysis. Arch Dermatol 1985;121:1548-9.
[Google Scholar]
|
| 83. |
Delattre JY, Safai B, Posner JB. Erythema multiforme and Stevens-Johnson syndrome in patients receiving cranial irradiation and phenytoin. Neurology 1988;38:194-8.
[Google Scholar]
|
| 84. |
Egan CA, Grant WJ, Morris SE, Saffle JR, Zone JJ. Plasmapheresis as an adjunct treatment in toxic epidermal necrolysis. J Am Acad Dermatol 1999;40:458-61.
[Google Scholar]
|
| 85. |
Bamichas G, Natse T, Christidou F, Stangou M, Karagianni A, Koukourikos S, et al. Plasma exchange in patients with toxic epidermal necrolysis. Ther Apher 2002;6:255-8.
[Google Scholar]
|
| 86. |
Trautmann A, Klein CE, Kämpgen E, Bröcker EB. Severe bullous drug reactions treated successfully with cyclophosphamide. Br J Dermatol 1998;139:1127-8.
[Google Scholar]
|
| 87. |
Walmsley SL, Khorasheh S, Singer J, Djurdjev O. A randomized trial of N-acetylcysteine for prevention of trimethoprim-sulfamethoxazole hypersensitivity reactions in Pneumocystis carinii pneumonia prophylaxis (CTN 057). Canadian HIV Trials Network 057 Study Group. J Acquir Immune Defic Syndr Hum Retrovirol 1998;19:498-505.
[Google Scholar]
|
| 88. |
Revuz J. New advances in severe adverse drug reactions. Dermatol Clin 2001;19:697-709, ix.
[Google Scholar]
|
| 89. |
de Sica-Chapman A, Williams G, Soni N, Bunker CB. Granulocyte colony-stimulating factor in toxic epidermal necrolysis (TEN) and Chelsea and Westminster TEN management protocol [corrected]. Br J Dermatol 2010;162:860-5.
[Google Scholar]
|
| 90. |
Goulden V, Goodfield MJ. Recombinant granulocyte colony-stimulating factor in the management of toxic epidermal necrolysis. Br J Dermatol 1996;135:305-6.
[Google Scholar]
|
| 91. |
Mahajan R, Kanwar AJ. Use of granulocyte colony-stimulating factor in the treatment of toxic epidermal necrolysis – Experience with 3 patients. Skinmed 2013;11:269-71.
[Google Scholar]
|
| 92. |
Wolkenstein P, Latarjet J, Roujeau JC, Duguet C, Boudeau S, Vaillant L, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet 1998;352:1586-9.
[Google Scholar]
|
| 93. |
Fischer M, Fiedler E, Marsch WC, Wohlrab J. Antitumour necrosis factor-alpha antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol 2002;146:707-9.
[Google Scholar]
|
| 94. |
Kreft B, Wohlrab J, Bramsiepe I, Eismann R, Winkler M, Marsch WC. Etoricoxib-induced toxic epidermal necrolysis: Successful treatment with infliximab. J Dermatol 2010;37:904-6.
[Google Scholar]
|
| 95. |
Gubinelli E, Canzona F, Tonanzi T, Raskovic D, Didona B. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol 2009;36:150-3.
[Google Scholar]
|
| 96. |
Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson syndrome and toxic epidermal necrolysis: Comparison with case-control analysis. Clin Pharmacol Ther 2010;88:60-8.
[Google Scholar]
|
| 97. |
Knight L, Todd G, Muloiwa R, Matjila M, Lehloenya RJ. Stevens Johnson syndrome and toxic epidermal necrolysis: Maternal and foetal outcomes in twenty-two consecutive pregnant HIV infected women. PLoS One 2015;10:e0135501.
[Google Scholar]
|
| 98. |
Struck MF, Illert T, Liss Y, Bosbach ID, Reichelt B, Steen M. Toxic epidermal necrolysis in pregnancy: Case report and review of the literature. J Burn Care Res 2010;31:816-21.
[Google Scholar]
|
| 99. |
Niemeijer IC, van Praag MC, van Gemund N. Relevance and consequences of erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in gynecology. Arch Gynecol Obstet 2009;280:851-4.
[Google Scholar]
|
| 100. |
Meneux E, Wolkenstein P, Haddad B, Roujeau JC, Revuz J, Paniel BJ. Vulvovaginal involvement in toxic epidermal necrolysis: A retrospective study of 40 cases. Obstet Gynecol 1998;91:283-7.
[Google Scholar]
|
| 101. |
Rodriguez G, Trent JT, Mirzabeigi M, Zaulyanov L, Bruce J, Vincek V. Toxic epidermal necrolysis in a mother and fetus. J Am Acad Dermatol 2006;55 5 Suppl: S96-8.
[Google Scholar]
|
| 102. |
Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis 2010;5:39.
[Google Scholar]
|
| 103. |
Pacheco H, Araujo T, Kerdel F. Toxic epidermal necrolysis in a pregnant, HIV-infected woman. Int J Dermatol 2002;41:600-1.
[Google Scholar]
|
| 104. |
Paziana K, Del Monaco M, Cardonick E, Moritz M, Keller M, Smith B, et al. Cyclosporin use during pregnancy. Drug Saf 2013;36:279-94.
[Google Scholar]
|
| 105. |
Forman R, Koren G, Shear NH. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in children: A review of 10 years' experience. Drug Saf 2002;25:965-72.
[Google Scholar]
|
| 106. |
Mayes T, Gottschlich M, Khoury J, Warner P, Kagan R. Energy requirements of pediatric patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Nutr Clin Pract 2008;23:547-50.
[Google Scholar]
|
| 107. |
Koh MJ, Tay YK. An update on Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Curr Opin Pediatr 2009;21:505-10.
[Google Scholar]
|
| 108. |
Rasmussen JE. Erythema multiforme in children. Response to treatment with systemic corticosteroids. Br J Dermatol 1976;95:181-6.
[Google Scholar]
|
| 109. |
Halebian P, Corder VJ, Herndon D, Shires GT. A burn center experience with toxic epidermal necrolysis. J Burn Care Rehabil 1983;4:176-83.
[Google Scholar]
|
| 110. |
Halebian PH, Corder VJ, Madden MR, Finklestein JL, Shires GT. Improved burn center survival of patients with toxic epidermal necrolysis managed without corticosteroids. Ann Surg 1986;204:503-12.
[Google Scholar]
|
| 111. |
Jones WG, Halebian P, Madden M, Finkelstein J, Goodwin CW. Drug-induced toxic epidermal necrolysis in children. J Pediatr Surg 1989;24:167-70.
[Google Scholar]
|
| 112. |
Halebian PH, Shires GT. Burn unit treatment of acute, severe exfoliating disorders. Annu Rev Med 1989;40:137-47.
[Google Scholar]
|
| 113. |
Kelemen JJ 3rd, Cioffi WG, McManus WF, Mason AD Jr., Pruitt BA Jr. Burn center care for patients with toxic epidermal necrolysis. J Am Coll Surg 1995;180:273-8.
[Google Scholar]
|
| 114. |
Sheridan RL, Weber JM, Schulz JT, Ryan CM, Low HM, Tompkins RG. Management of severe toxic epidermal necrolysis in children. J Burn Care Rehabil 1999;20:497-500.
[Google Scholar]
|
| 115. |
Ginsburg CM. Stevens-Johnson syndrome in children. Pediatr Infect Dis 1982;1:155-8.
[Google Scholar]
|
| 116. |
Wolf R, Davidovici BB. Severe acute adverse cutaneous drug reactions I: Stevens-Johnson syndrome and toxic epidermal necrolysis. In: Wolf R, Davidovici BB, Parish LJ, Parish LC, editors. Emergency Dermatology. New Delhi: Cambridge University Press India Pvt. Ltd.; 2011. p. 154-67.
[Google Scholar]
|
Fulltext Views
48,710
PDF downloads
8,725





