Translate this page into:
32 P-patch contact brachyradiotherapy in the management of recalcitrant keloids and hypertrophic scars
2 Radioisotope Laboratory, Physics Department, School of Pharmacy and Biochemistry, University of Buenos Aires, Buenos Aires, Argentina
Correspondence Address:
M J Salgueiro
Radioisotope Laboratory, Physics Department, School of Pharmacy and Biochemistry, University of Buenos Aires, Junin 956 Piso Bajo, 1113-Buenos Aires
Argentina
| How to cite this article: Vivante H, Salgueiro M J, Ughetti R, Nicolini J, Zubillaga M. 32 P-patch contact brachyradiotherapy in the management of recalcitrant keloids and hypertrophic scars. Indian J Dermatol Venereol Leprol 2007;73:336-339 |
Abstract
Keloids are the result of excessive fibroblast proliferation and then over-abundant collagen deposition. There is no method able to guarantee absolute success in the therapeutic approach to keloids. Our case report involves a female patient with six lesions treated with a 32 P-patch bracyradiotherapy. Pre-treatment and adjuvant treatment of the lesions were performed with thiomucase, 5-fluoruracil, procaine and triamcinolone. Taking into account the activity contained in each of the patches and the total radiation dose to be administered according to clinical practice, dosimetric calculations were done for each lesion. Seperate silicone patches with chromic [ 32 P] phophate were designed for each lesion based on these calculations. Total remission was achieved in three treated lesions. The other lesions did not achieve total remission yet, but their sizes are diminishing. The differences observed in treatment outcome may be related with lesion features, adjuvant treatments and/or treatment schedule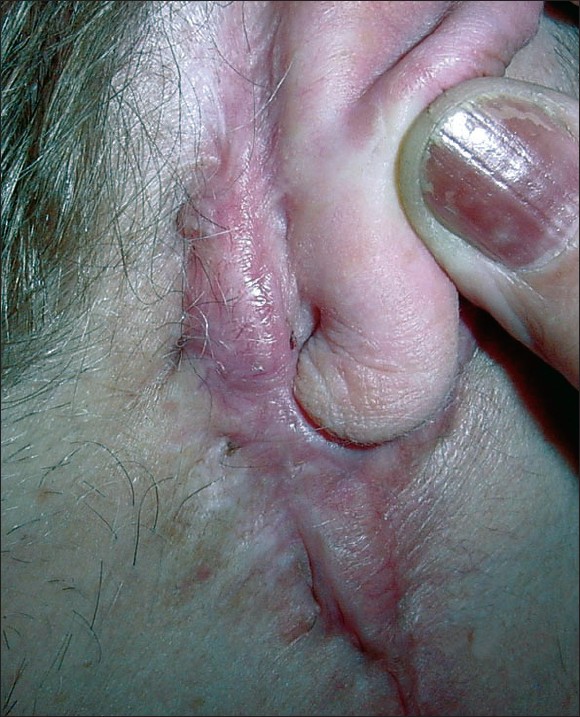 |
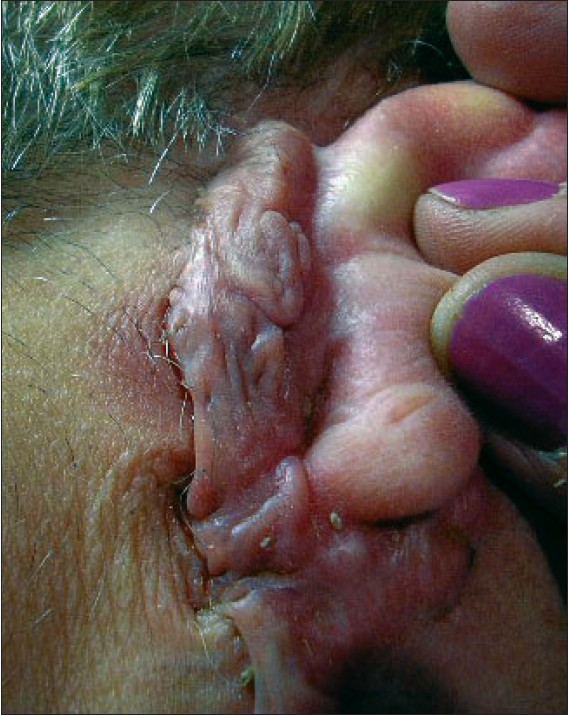
| Figure 2: Evolution of Qra after 8 months of 32P-patch treatment |
 |
| Figure 2: Evolution of Qra after 8 months of 32P-patch treatment |
 |
| Figure 1: Size and shape of the lesion behind the ear (only Qra is shown) previous to Qra treatment with 32P-patch |
 |
| Figure 1: Size and shape of the lesion behind the ear (only Qra is shown) previous to Qra treatment with 32P-patch |
Introduction
Keloids are the result of excessive fibroblast proliferation and then over-abundant collagen deposition. In spite of the several treatments modalities available, there is no method able to guarantee absolute success in the therapeutic approach to keloids. Surgical resection alone has been shown to result in a recurrence rate of 50-80% of the cases. Other treatments in current practice are application of silicone gel, intralesional injections of steroids or 5-flurouracil, pressure dressing therapy and ablatve therapies like cryotherapy, electrotherapy or radiation therapy. Nevertheless, post-operative radiation therapy appeared as one of the treatment modalities which has reduced the keloid formation substantially as well as radiation therapy in unresectable bulky symptomatic keloids. [1],[2],[3],[4],[5],[6],[7] Radiation therapy may be administered as teletherapy or brachytherapy. In this way, we designed and used a silicon 32 P-patch in order to administer a contact brachytherapy treatment. 32 P, which has a half life of 14 days, is a pure beta emitter with maximum beta energy of 1.7 MeV and a maximum range in soft tissue of 7.5 mm (average 3-4 mm). This high stopping power allows the local treatment of the lesions with minimal or absence of damage to normal surrounding and underlying tissue.
Our case report involves a female patient with six lesions, two keloids (Q1 and Qra) and four hypertrophic scars (Q2, Q3, Qsa and Qfa), treated with a silicon patch with 32 P.
Case Report
Patient: A female patient (81 years) presented with six lesions (two keloids and four hypertrophic scars). All lesions developed after the resection of four moles in 1994. Three of the moles were on the back and the other one under the right ear. The lesions of the back were placed in D7 (Q1), L2 (Q2) and L4 (Q3). Q1 was a keloid and Q2 and Q3 were hypertrophic scars. The lesion under the right ear was originally 11 mm diameter, but subsequent treatments (cryosurgery, intralesional steroid injections and surgeries) in order to achieve complete remission failed and contributed to increase its size up to 110 mm in length, 11 mm superior width and 2 mm inferior width [Figure - 1]. Therefore, we assumed this lesion as if they were three, because of their characteristics: a keloid (behind the pinna, Qra) and two hypertrophic scars (under the pinna and behind the ear lobe, Qsa; and next to the ear lobe in the face, Qfa). With regard to the lesions of the back, only Q1 was previously treated (in the same way as Qra), and similar results were obtained as the original diameter of 10 mm increased up to 44 mm in length, 16 mm inferior width and 12 mm superior width. The other two lesions Q2 and Q3 were not treated and remained unchanged since 1994 with a size of 5.5 mm and 11 mm diameter, respectively. The patients informed signed consent was obtained after explaining her all procedures, alternatives, benefits and risks.
Treatment: Lesions on the back were first treated in order to evaluate cosmetic results. Lesion of the ear was subdivided in three regions as Qra (behind the pinna), Qsa (under the pinna and behind the ear lobe) and Qfa (face).
Measurements of the lesions were taken and the patches were specially designed for their application on each lesion surface with minimal contact with the normal surrounding tissue. Additionally, each patch was shielded on the external face, with aluminium of 2 mm of thickness in order to protect the physician and the patient.
Q1, Q2, Q3 and Qra were treated with a single dose scheme for a total dose of 40 Gy with time exposition of 41, 59, 45 and 20 hours, respectively. The four lesions received pre-treatment with an intralesional combination of triamcinolone, procaine and 5-fluoruracil with additional thiomucase for Qra. The total activity and total surface of patches were 1.04 mCi and 10.26 cm 2 for Q1; 0.17 mCi and 2.40 cm 2 for Q2; 0.87 mCi and 9.50 cm 2 for Q3 and 2.83 mCi and 13.90 cm 2 for Qra.
Qsa and Qfa were treated with a fractionated dose scheme for a total dose of 30 Gy. Both lesions received pre-treatment with an intralesional combination of procaine, 5-fluoruracil and thiomucase. Fractionated scheme consisted for Qfa of one session of 2 Gy and seven sessions of 4 Gy each during an overall treatment time of 17 days. Qsa was treated along six sessions of 5 Gy each during an overall treatment time of 14 days. Total surface of the patches were 3.80 cm 2 and 2.50 cm 2 and initial activities were 1.4 mCi and 0.69 mCi for Qfa and Qsa, respectively.
Preparation of the 32 P-patch: The patches were prepared by Laboratorios Bacon S.A.I.C. using chromic [ 32 P]-phosphate (30-70 nm) as proposed by Anghileri and Marques and silicone (Silastic ®J-White 80, Dow Corning, The Dow Chemical Company and Corning, Incorporated, Michigan, USA). Polymerization time was variable (1-4 hours) depending on the final activity of the patches.
Measurement of activity concentration of 32 P-patches . A sample of each patch was dissolved with 5 mL of hyamine hydroxide (MP Biomedicals, LLC, USA) at room temperature overnight. An aliquot exactly measured was added to a vial containing 3 ml of a complete phase combining system for liquid scintillation counting (PCS ® Amersham Biosciences, USA). The activity measurement was performed in a liquid scintillation counter (Wallac 1410 Liquid Scintillation Counter, Pharmacia Wallac OY, Finland) according to the 32 P protocol, with a relative error < 1%.
Dosimetric calculations : Taking into account the activity contained in the patch, the total radiation dose to administer according to clinical practice, and a model of beta dosimetry of a source in contact with the skin, dosimetric calculations were done for each lesion using the following equation derived from the MIRD dose system:
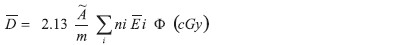
The result expresses the average absorbed dose in a tissue depth equal to the maximum range of 32 P in water considering a plane source in contact with the skin. [8],[9]
Q1 achieved total remission within 2 months of treatment. Erythema and hyperpigmentation developed two days after patch application as a consequence of radiation therapy, but the former was treated with topical steroids with good response within 24 hours and the latter is gradually disappearing. Q2 and Q3 reduced their size up to 50% 1 month after radiation therapy and adjuvant treatment with intralesional injections of thiomucase, procaine and 5-fluoruracil was administered 3 months after patch application. Six months later, Q2 achieved a partial remission by reducing its size up to 80% and Q3 achieved total remission. Finally, Q2 achieved total remission after 11 months of radiotherapy with 32 P-patch. Qra reduced its size up to 50% of the original within 1 week after therapy with 32 P and a partial remission of 70% was achieved with no complications 4 weeks after patch application. Nevertheless, infection developed 6 weeks after radiation therapy. Patient was successfully treated with antibiotics during 7 days, but infection recurred 15 days after. The balance between remission and recurrence of infection continued over three months until an anti-streptococcal vaccine was locally applied with excellent results. The evolution of Qra after 8 months of patch application is shown in [Figure - 2], with a reduction of 80% of its original size. Qfa evolved without complications. Erythema developed 1 week after radiotherapy but it was overcome without additional treatment. Reduction of size occurred to almost 50%, and the lesion is now smooth without anfractuosities. Qsa is reducing its thickness slowly. Erythema also developed 1 week after 32 P therapy but it evolved without complications.
Discussion
This case report confirms the efficacy of contact brachytherapy treatment of keloids and hypertrophic scars. Total remission was achieved in three treated lesions within 2, 6 and 8 months post-treatment (Q1, Q3 and Q2 respectively) with no recurrence at 11 months of follow-up. The other lesions did not achieve total remission yet, but their sizes are diminishing (90% after 8 months for Qra and 50% after 4 months for Qfa). With regard to Qsa, its evolution is favourable after 10 days of radiation therapy. The differences observed in treatment outcome may be related with lesion features (extensive lesions, resistant, relapsing, etc.), adjuvant treatments and/or treatment schedule. Nevertheless, it has to be evaluated as positive, considering the type of lesions, the fact that adjuvant therapies that had previously proved ineffective were able to block keloid growth or contribute to total remission when combined with this treatment modality.
To our knowledge, this is the first report about a contact brachytherapy treatment of keloids and hypertrophic scars with a silicon 32 P-patch although there are many other reports in literature confirming the usefulness and safety of other radiotherapy modalities for keloids treatment. [4],[5],[6] Radiation therapy has been employed to treat skin diseases during many years since its discovery in the early 1900`s. Along these decades, the experience in preclinical investigation and clinical applications yielded interesting results which allowed to improve the treatment protocols of many health conditions including keloids. However, the safety issue may be the main cause of hesitation when considering radiotherapy as a possible treatment because of the risk of radiation-induced malignancies. In this way, reports of a population of exposed cases and matched controls where the risks are compared during a long-term follow up may be helpful to establish any specific association. [6] Unfortunately, these kinds of studies are very few and cancer is a common disease. Therefore, (a) as the safety is not reliably demonstrated. The radiotherapist must explain this to the patient as part of the consent procedure and (b) radiotherapy must be administered in compliance with strict protection rules by authorized professionals according to the local nuclear authorities.
Brachytherapy is by definition a very local radiation. The 32 P-patch fulfilled this requirement because of the physical characteristics of 32 P and the patch design with regard to its shape and shielding. Additionally, these features contribute to radiation safety for both the patient and the physician.
| 1. |
Brissett AE, Sherris DA. Scar contractures, hypertrophic scars, and keloids. Facial Plast Surg 2001;17:263-72.
[Google Scholar]
|
| 2. |
Kal HB, Veen RE. Biologically effective doses of postoperative radiotherapy in the prevention of keloids. Strahlenther Onkol 2005;181:717-23.
[Google Scholar]
|
| 3. |
Malaker K, Vijayraghavan K, Hodson I, Al Yafi T. Retrospective analysis of treatment of unresectable keloids with primary radiation over 25 years. Clin Oncol (R Coll Radiol) 2004;16:290-8.
[Google Scholar]
|
| 4. |
Caccialanza M, Piccinno R, Schiera A. Postoperative radiotherapy of keloids: A twenty-year experience. Eur J Dermatol 2002;12: 58-62.
[Google Scholar]
|
| 5. |
Garg M, Weiss P, Sharma A, Gorla G, Jaggernauth W, Yaparpalvi R, et al . Adjuvant high dose rate brachytherapy (Ir-192) in the management of keloids which have recurred after surgical excision and external radiation. Radiother Oncol 2004;73:233-6.
[Google Scholar]
|
| 6. |
Ragoowansi R, Corne P, Moss A, Glees J. Treatment of keloids by surgical excision and immediate postoperative single-fraction radiotherapy. Plast Reconstr Surg 2003;111:1853-9.
[Google Scholar]
|
| 7. |
Dinh Q, Veness M, Richards S. Role of adjuvant radiotherapy in recurrent earlobe keloids. Australas Dermatol 2004;45:162-6.
[Google Scholar]
|
| 8. |
Harbert JC. Nuclear Medicine Therapy. Ed Thieme Medical Publishers, Inc. NY, USA, 1987.
[Google Scholar]
|
| 9. |
MIRD Committee. MIRD primer for absorbed dose calculations. Ed The Society of Nuclear Medicine, Inc. NY, USA, 1988.
[Google Scholar]
|
Fulltext Views
3,352
PDF downloads
2,717





