Translate this page into:
Primary extramammary Paget's disease with extensive skeletal metastases
2 Department of Pathology, Apollo Hospitals, Hyderabad, India
3 Department of Dermatology, Image Hospital, Hyderabad, Andhra Pradesh, India
Correspondence Address:
Indukooru Subrayalu Reddy
A-8, Anand Sheel Enclave, Nandi Nagar, Road No. 14, Banjara Hills, Hyderabad - 500 034, Andhra Pradesh
India
| How to cite this article: Reddy IS, Swain M, Gowrishankar S, Murthy DN. Primary extramammary Paget's disease with extensive skeletal metastases. Indian J Dermatol Venereol Leprol 2012;78:89-92 |
Abstract
Extramammary Paget's disease (EMPD) is an uncommon malignancy that is most commonly seen in the vulval area in postmenopausal women. Pruritus is the predominant symptom. The clinical presentation can be so nonspecific that it can be misdiagnosed as an inflammatory or infective condition. We report an elderly male patient with EMPD over the pubic area, which remained asymptomatic for 5 years; he presented with severe low backache of 5 months' duration. Skin biopsy and immunohistochemistry showed the typical epidermal changes and deep dermal invasion. Positron emission tomography scan revealed involvement of regional lymph nodes as well as extensive skeletal metastases.Introduction
Extramammary Paget′s disease (EMPD) was originally described by Crocker in 1989 in a male patient in whom there was involvement of the scrotum and penis. [1] It is a rare cutaneous malignancy, occurring in sites rich in apocrine glands. EMPD presents as a pruritic, slowly spreading, erythematous, eczematoid plaque. The precise pathogenesis and cell of origin of EMPD remains controversial. Apocrine and eccrine ductal and glandular cells, pluripotent keratinocyte stem cells, and Toker cells have all been considered as possible cells of origin of EMPD. [2],[3] Based on the pathogenic mechanisms, EMPD is classified as primary (intraepidermal) or secondary. Patients with primary EMPD carry a better prognosis. [4] Factors such as dermal invasion, presence of nodules in the skin, and lymph node metastasis are associated with poor prognosis. We report a patient of EMPD with dermal invasion and extensive skeletal metastases.
Case Report
A 60-year-old male presented with severe backache since 5 months. Since the last 5 years he had an asymptomatic, slowly progressing, ill-defined, hypopigmented patch involving the pubic area and dorsal aspect of the root of the penis. He had also developed an asymptomatic, irregular, beefy-red erosion over the left side of the pubic area since 2 years [Figure - 1]. This erosion had developed over the preexisting hypopigmented patch. The margin around the erosion was indurated and firm. A few comedo-like plugs were present over the hypopigmented patch. The inguinal lymph nodes were not enlarged. He denied history of any genitourinary or lower gastrointestinal tract symptoms. The clinical differential diagnoses considered were Bowen′s disease, basal cell carcinoma, and lupus vulgaris.
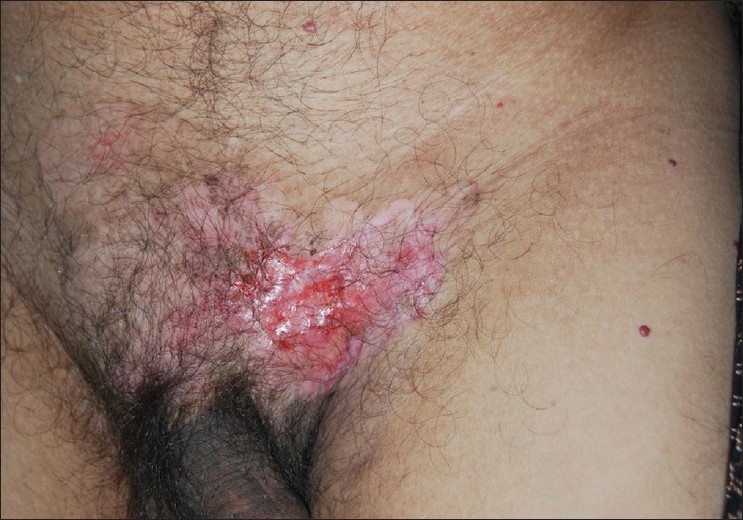 |
| Figure 1: Hypopigmented patch and beefy-red erosion over the pubic area |
Punch biopsies were obtained from the beefy-red erosion and from the indurated area. The hematoxylin and eosin-stained sections from the beefy-red erosion showed scattered large cells with vesicular nuclei having nucleoli and a broad rim of clear cytoplasm infiltrating through the entire thickness of the epidermis [Figure - 2]a. Some of the cells had an eccentric nucleus, giving a ′signet ring′ appearance [Figure - 2]b. The indurated area showed numerous atypical cells with prominent nuclei, arranged in a linear cord in an ′Indian-file′ pattern between the collagen bundles of the reticular dermis [Figure - 2]c. Periodic acid Schiff (PAS) stain and carcinoembryonic antigen (CEA) immune stain showed strong cytoplasmic positivity in the infiltrating cells [Figure - 3]a and b. These cells also demonstrated strong cytoplasmic positivity for cytokeratin-7 (CK-7) and gross cystic disease fluid protein-15 (GCDFP-15) and were negative for cytokeratin-20 (CK-20) [Figure - 4]a-c.
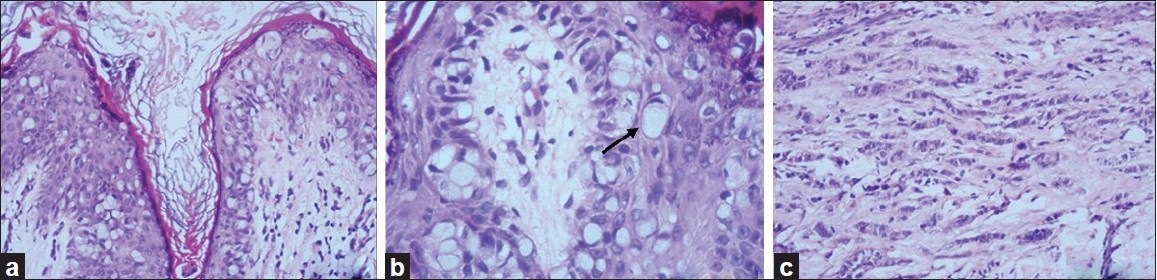 |
| Figure 2: (a) Low-power view showing intraepidermal Paget cells (H and E, ×100); (b) high-power view with signet-ring cells (arrow) (H and E, ×400); (c) linear cords of tumor cells in an 'Indian-file' pattern in the reticular dermis (H and E, ×100) |
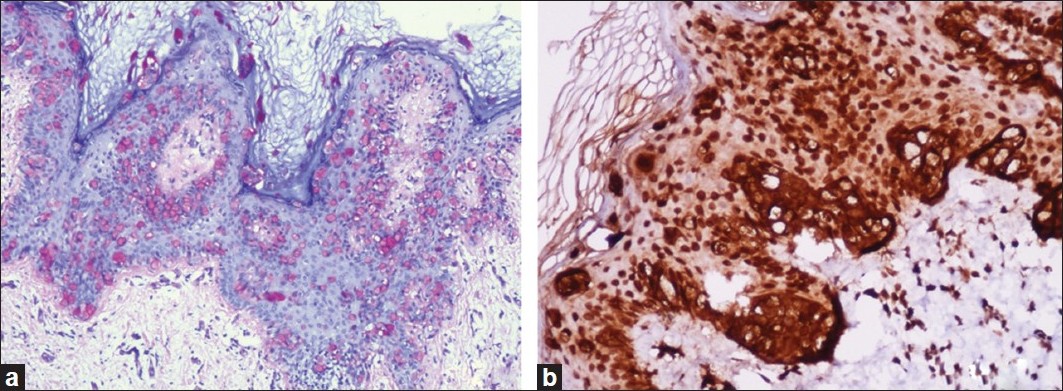 |
| Figure 3: (a) Paget cells showing intracytoplasmic PAS positivity (PAS, ×200); (b) CEA immune stain showing strong cytoplasmic positivity in the tumor cells (IHC, ×200) |
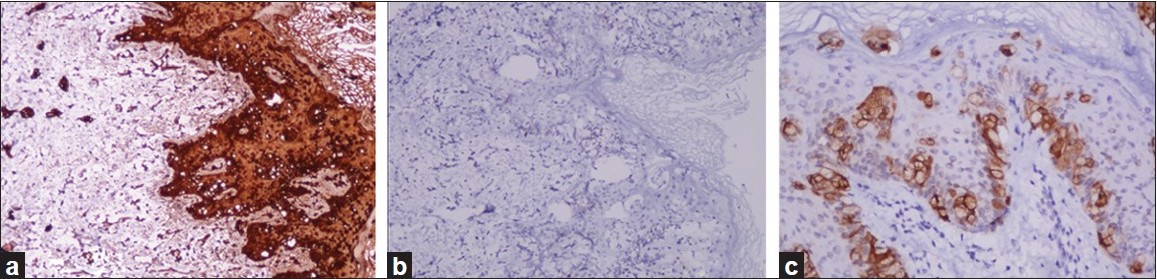 |
| Figure 4: (a) CK-7 positivity in tumor cells (IHC, ×200); (b) tumor cells negative for CK-20 (IHC, ×200); (c) GCDFP-15 positivity in scattered tumor cells (IHC, ×200) |
Whole-body positron emission tomography (PET) scan using 18F fluorodeoxyglucose (FDG) demonstrated mild uptake at the skin lesion and also the involvement of bilateral inguinal lymph nodes and external iliac nodes. Foci of FDG-avid hypercatabolic spots were seen in almost all the vertebrae, sternum, ribs, pelvic girdles, and the proximal humeri and femora [Figure - 5]a and b.
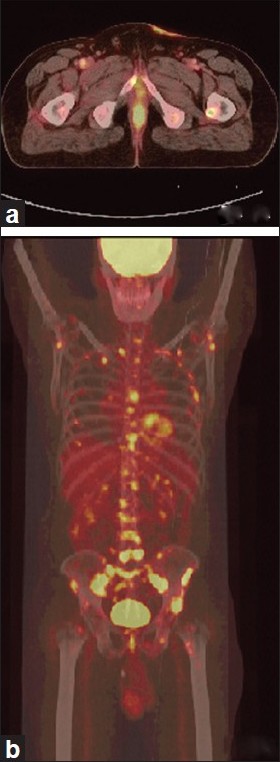 |
| Figure 5: (a) PET-CT showing FDG uptake as irregular skin thickening in the left pubic region; (b) Foci of abnormal FDG uptake in all vertebrae, sternum, ribs, limb girdle bones, and proximal femora and humeri |
Based on the long history, clinical examination, routine histopathology findings, and immunohistochemistry, the diagnosis of primary EMPD was made. FDG-PET scan confirmed nodal and skeletal metastases. In view of the extensive metastases, surgical management was ruled out. The patient refused chemotherapy and was given radiation to the lower back and intravenous zoledronic acid for the pain relief.
Discussion
The current theory is that primary EMPD arises as an intraepidermal neoplasm, in most cases from the intraepidermal portion of an apocrine sweat duct or from pluripotent keratinocyte stem cells. [2] This form is not associated with an underlying adenocarcinoma. The prognosis for this form of EMPD is excellent. Rarely, primary EMPD can become invasive, infiltrating the dermis and even metastasizing to the regional lymph nodes and to distant sites. [5] In contrast, secondary EMPD is due to an epidermotropic spread of malignant cells from an underlying adenocarcinoma in a dermal adnexal gland or due to contiguous spread from adjacent genitourinary or lower gastrointestinal tract epithelium. [6] The occasional co-occurrence of EMPD and mammary Paget′s disease suggest that oncogenic stimuli induce multicentric malignant changes in intraepidermal, adnexal, and distant anatomic areas. [7]
The pattern of cytokeratin expression may help in predicting the presence or absence of associated internal malignancy. [8] Patients with underlying lower gastrointestinal tract, especially colonic malignancy show CK-7 negativity and CK-20 positivity. [8] GCDFP-15 is strongly expressed in patients of EMPD without an underlying internal malignancy. [9] Primary (intraepidermal) EMPD shows CK-7 and GCDFP-15 positivity and CK-20 negativity [10] as was seen in this case. Positive staining with CEA is more in favor of EMPD, while negative staining seems to be associated more frequently with underlying carcinoma. [11] PAS positivity is due to the presence of sialomucin, which is present in large quantities in patients with EMPD, displacing the nuclei of Paget cells to the periphery and giving rise to the ′signet ring′ appearance. [12] Cho et al. reported seven patients with EMPD in whom whole-body 18F-FDG-PET scan showed mild FDG uptake at the primary site in the skin in four patients. Among these four patients, three showed dermal invasion and two patients showed multiple hypermetabolic foci of skeletal metastases and lymph node involvement. [13]
Patients with intraepidermal and minimally invasive (up to the level of the papillary dermis) EMPD carry a good prognosis. Clinical presence of a nodule in the primary lesion, regional lymph node involvement, and histopathological evidence of dermal invasion are indicators of poor prognosis. Hatta and colleagues have proposed a lymph node staging system where ′N0′ implies no lymph node involvement, ′N1′ unilateral involvement, and ′N2′ bilateral or distant metastatic disease. [4]
Various treatment modalities are advocated for EMPD. Surgery is considered the mainstay of treatment, especially in patients with primary intraepidermal disease. Other modalities of treatment are CO 2 and Nd:YAG lasers and photodynamic therapy. Topical treatment using 5-flurouracil, 3.5% bleomycin, and imiquimod have been reported to be effective. Many anecdotal reports have been published of various chemotherapeutic regimes in locally advanced and metastatic EMPD; these include 5-fluorouracil alone and combinations of 5-fluorouracil/ mitomycin C, carboplatin/ 5-fluorouracil, vincristine/ cisplatin/ 5-fluorouracil.
We report this case because of its rarity and because of the extensive skeletal metastases, which is also unusual. We wish to emphasize the importance of early biopsy for any persistent and treatment-resistant skin lesion over the external genital and perianal region.
| 1. |
Crocker HR. Paget's disease affecting the scrotum and penis. Trans Pathol Soc Lond 1888-1889;40:187-91.
[Google Scholar]
|
| 2. |
Lloyd J, Flanagan AM. Mammary and extramammary Paget's disease. J Clin Pathol 2000;53:742-9.
[Google Scholar]
|
| 3. |
Willman JH, Golitz LE, Fitzpatrick JE. Vulvar clear cells of Toker: Precursors of extra mammary Paget's disease. Am J Dermatopathol 2005;27:185-8.
[Google Scholar]
|
| 4. |
Hatta N, Yamada M, Hirano T, Fujimoto A, Morita R. Extramammary Paget's disease: Treatment, prognostic factors and outcome in 76 patients. Br J Dermatol 2008;158:313-8.
[Google Scholar]
|
| 5. |
Cappucini F, Tewari K, Rogers LW, Di Saia PJ. Extramammary Paget's disease of the vulva: Metastases to the bone marrow in the absence of an underlying adenocarcinoma - Case report and literature review. Gynecol Oncol 1997;66:146-50.
[Google Scholar]
|
| 6. |
Shepherd V, Davidson EJ, Davies-Humphreys J. Extramammary Paget's disease. BJOG 2005;112:273-9.
[Google Scholar]
|
| 7. |
Kanitakis J. Mammary and extramammary Paget's disease. J Eur Acad Dermatol Venereol 2007;21:581-90.
[Google Scholar]
|
| 8. |
Goldblum JR, Hart WR. Vulvar Paget's disease: A clinicopathologic and immunofluroscent study of 19 cases. Am J Surg Pathol 1997;21:1178-87.
[Google Scholar]
|
| 9. |
Kohler S, Smoller BR. Gross cystic disease fluid protein-15 reactivity in extramammary Paget's disease with or without internal malignancy. Am J Dermatopathol 1996;11:79-92.
[Google Scholar]
|
| 10. |
Lam C, Funaro D. Extramammary Paget's disease: Summary of current knowledge. Dermatol Clin 2010;28:807-26.
[Google Scholar]
|
| 11. |
Battles OE, Page DL, Johnson JE. Cytokeratins, CEA and mucin histochemistry in the diagnosis and characterisation of extramammary Paget's disease. Am J Clin Pathol 1997;108:6-12.
[Google Scholar]
|
| 12. |
Goldblum JR, Hart WR. Perianal Paget's disease: A histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol 1998;2:170-9.
[Google Scholar]
|
| 13. |
Cho SB, Yum M, Lee MG, Chung KY. Variable patterns of positron emission tomography in the assessment of patients with extramammary Paget's disease. J Am Acad Dermatol 2005;52:353-5.
[Google Scholar]
|
Fulltext Views
5,104
PDF downloads
1,649





