Translate this page into:
Comparative efficacy and safety of topical permethrin, topical ivermectin, and oral ivermectin in patients of uncomplicated scabies
2 NHL Municipal Medical College, Ahmedabad, Gujarat, India
3 Department of Dermatology, C. U. Shah Medical College, Surendranagar, Gujarat, India
Correspondence Address:
Sunita B Chhaiya
Department of Pharmacology, C. U. Shah Medical College, Dudhrej Road, Surendranagar, Gujarat
India
| How to cite this article: Chhaiya SB, Patel VJ, Dave JN, Mehta DS, Shah HA. Comparative efficacy and safety of topical permethrin, topical ivermectin, and oral ivermectin in patients of uncomplicated scabies. Indian J Dermatol Venereol Leprol 2012;78:605-610 |
Abstract
Background: Ivermectin has opened a new era in the management of scabies as orally effective drug. However, topical route has been little explored for ivermectin. Aims: To compare the efficacy and safety of topical permethrin, oral ivermectin, and topical ivermectin in the treatment of uncomplicated scabies. Methods: This was an open-label, randomized, comparative, parallel clinical trial conducted in 315 patients, randomly allocated to 3 groups. First group received permethrin 5% cream as single application, second group received tablet ivermectin 200 mcg/kg as single dose, and third group received ivermectin 1% lotion as single application. All the patients received anti-histaminic for pruritus. The patients were followed up at intervals of 1, 2, 3, and 4 weeks. If there were no signs of cure, the same intervention was repeated at each follow up. Primary efficacy variable was clinical cure of lesions. Statistical analysis was done by chi square test and one way ANOVA test using SPSS version 12. Results: At the end of first week, cure rate was 74.8% in permethrin group, 30% in oral ivermectin group, and 69.3% in topical ivermectin group (P < 0.05). At the end of second week, cure rate was 99% in permethrin group, 63% in oral ivermectin group, and 100% in topical ivermectin group (P < 0.05). At the end of third week, 100% cure rate was observed in permethrin and topical ivermectin group while 99% in oral ivermectin group (P = 0.367). No serious adverse events were observed. Conclusions: Permethrin and topical ivermectin were equally effective against scabies while oral ivermectin was significantly less effective up to 2 weeks. Topical ivermectin can be used as an alternative to permethrin.Introduction
Scabies is a common parasitic infection caused by the mite Sarcoptes scabiei var. hominis. It is a major global health problem in many indigenous and third world communities. [1] It has been estimated that 300 million people suffer from scabies infestation at any one time. Scabies is an important disease of children, but it occurs in both sexes at all ages, in all ethnic groups and in all socio-economic levels. [2] Different therapies for scabies consist of topical anti-scabietics such as benzyl benzoate, crotamiton, lindane, and permethrin. At present, permethrin 5% has become the anti-scabietic of choice in United States because of report of resistance to and central nervous system toxicity to lindane. [3] Ivermectin is an anti-parasitic agent, effective against a variety of endoparasites and ectoparasites. [4] Initial reports have highlighted the utility of oral ivermectin in the treatment of scabies. [5],[6],[7],[8],[9],[10],[11],[12],[ 13] Topical ivermectin has also been shown to be effective in some studies. [14],[15],[16] However, these studies were non-comparative and were conducted with small number of patients. Hence, this randomized clinical study was planned and conducted to generate more data regarding efficacy and safety of topical ivermectin in treatment of scabies and to compare with oral ivermectin and currently available first line drug permethrin.
Methods
This was an open-label, randomized, comparative, parallel group clinical trial. The study was approved by the institutional ethics committee and was performed as per the ICH-GCP guidelines and GCP guidelines by government of India. From June 2007 to January 2009, the patients willing to participate were enrolled using following inclusion and exclusion criteria.
Inclusion criteria
1. Patients of either sex aged 5 to 80 years with clinically-diagnosed scabies, 2. Presence of typical scabietic lesions like papules, nodules, or vesicles at classical sites, 3. Presence of classical burrows on clinical examination, 4. Nocturnal pruritus, 5. History of involvement of family member or similar symptoms in contacts, 6. Microscopically-diagnosed scabies (demonstration of egg, larvae, mite, or fecal material), 7. Patients whose microscopic examination was negative, their inclusion in study was based on clinical criteria, for that patient had to satisfy at least 3 out of 4 inclusion criteria (inclusion criteria no. 2 to 5), 8. Patients who were willing to participate and give written informed consent.
Exclusion criteria
1. Patient treated with any topical scabicidal therapy in the month before entry, 2. Patients taking any topical or systemic antibiotic therapy in the week before entry into the study, 3. Immunologically-compromised patients, 4. Having scabies with atypical presentation like crusted scabies or scabies incognito, 5. Patients with secondary bacterial infection, 6. History of allergy to any of the study drugs, 7. Blood pressure < 100/60 mm Hg, 8. Pregnancy in women and lactating mothers.
Severity of lesions was clinically graded on a scale of 0 to 3. Grade 0 - free of lesions (no lesions), Grade 1 - 10 or fewer lesions (mild), Grade 2 - 11 to 49 lesions (moderate), Grade 3 - 50 or more lesions (severe). Itching was graded on a scale of 0 to 3 on basis of severity. Grade 0 - no itching, Grade 1 - mild itching, Grade 2 - moderate itching, Grade 3 - severe / intense itching.
Sample size
Sample size was calculated by using sample size calculator (Raosoft, 2004). Considering alpha error at 5%, confidence level at 90%, and sample size of 267 patients was obtained. Considering dropout rate 10%, sample size of 294 was finalized.
Randomization
The 315 enrolled participants were allocated to any 1 of the 3 treatment groups according to random allocation number generated through computer by using complete randomized design for 3 treatment groups [Figure - 1].
 |
| Figure 1: Flow chart of patients in the trial |
Interventions
Permethrin 5% Cream
Permethrin 5% cream was supplied by Shalaks Pharmaceutical Ltd., New Delhi. The patients included in this group were given permethrin 5% cream along with printed information sheet in the local vernacular language. They were asked to apply the cream to whole body covering neck to toe. They were explained that the cream must remain in contact with the skin for at least 8 hours. They were advised to take bath with warm water not earlier than 8 hours after application.
Tablet ivermectin
Tablet ivermectin was supplied by Shalaks Pharmaceutical Ltd., New Delhi. The patients included in this group were given tablet ivermectin orally in the dose of 200 mcg/kg as a single dose to be self-administered along with printed information sheet in the local vernacular language.
Ivermectin lotion 1%
Ivermectin powder and lotion base were supplied by Shalaks Pharmaceutical Ltd., New Delhi. Ivermectin 1% lotion was prepared by mixing 1 gram ivermectin powder with 100 ml lotion base (contains propylene glycol). It was prepared according to the requirement. Proper aseptic measures were observed during the preparation. The patients included in this group were given ivermectin 1% lotion along with printed information sheet in the local vernacular language. The information sheet contained details regarding application of drug and other instructions. They were asked to apply the lotion to all the affected sites. They were explained that the lotion must remain in contact with the skin for at least 8 hours. They were advised to take bath with warm water not earlier than 8 hours after application.
Antihistaminic
All patients received orally hydroxyzine 10 mg or 25 mg twice-daily for symptomatic treatment of pruritus.
Assessment of patients
Patients were followed up at the end of first, second, third, and fourth week to assess compliance and to examine the patient to evaluate efficacy and safety.
Primary end point was clinical cure of scabietic lesions, and Secondary end point was complete relief of pruritus. At each of the 4 visits, examination of the entire body surface was performed. All remaining suspected scabies lesions were examined and compared with baseline clinical grading score and itching grading score. The patients were asked for any adverse event occurring during previous week. The cured participants were prescribed anti-histaminic for further 1 week if itching grade was severe or moderate. If the patients had mild itching, no anti-histaminic was prescribed. The participants who were not cured were prescribed repeat intervention along with anti-histaminic. All the participants were again called for follow-up visit after 1 week. The participants who were not cured at the end of third week were switched over to standard treatment with 5% permethrin.
Statistical analysis
It was done by using SPSS version 12.0. Differences in proportions were compared with the Chi-square test. Statistical analysis of efficacy was done by Chi-square test and one way ANOVA test. Post hoc test was used to identify significant difference between the groups for which LSD (Least Significant Differences) test was used. P values < 0.05 were considered significant.
Results
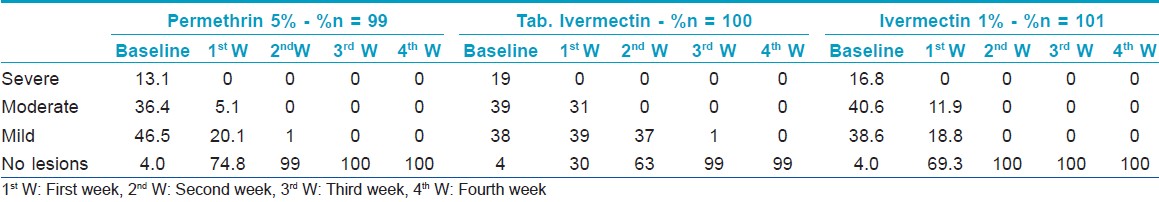
Total 315 patients were enrolled in the study and randomly allocated to 3 treatment groups, each group having 105 patients. There was no significant difference between the 3 study groups at baseline in demographic and clinical characteristics [Table - 1]. At each follow-up visit, patients were assessed clinically as only 22.5% patients were positive on microscopic examination at initial visit.

Clinical cure rate at different visits shows [Table - 2] that there is significant difference between tablet ivermectin group and the other 2 groups up to 2 weeks (P < 0.05). At the end of third week, there was no statistically significant difference between the 3 groups (P = 0.367). [Figure - 2]
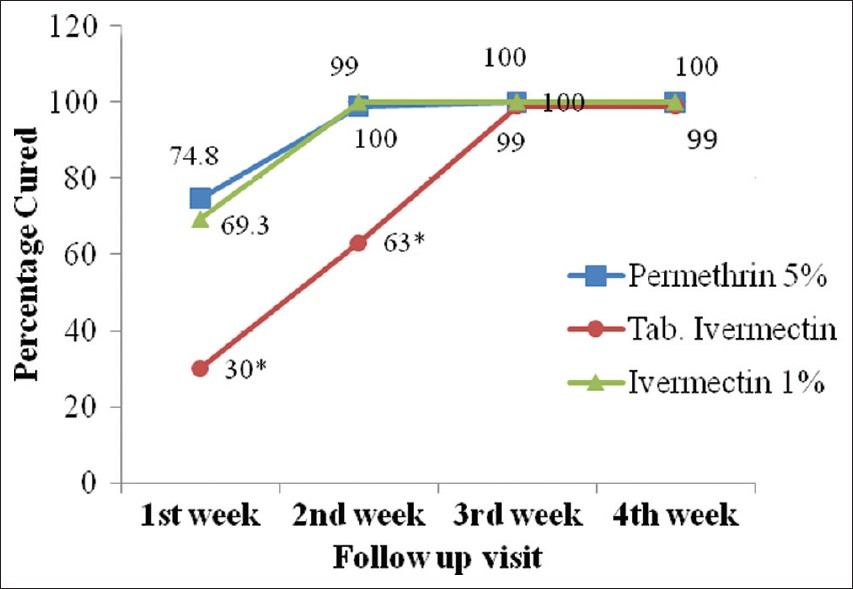 |
| Figure 2: Clinical cure rate at different visits.* shows P < 0.05 for tab. ivermectin compared to permethrin cream and ivermectin lotion at the end of first and second week using ANOVA followed by post hoc test (LSD) |
Considering pruritus as main symptom, the anti-scabies medication, which alleviates it, has greater acceptance in clinical practice. Improvement in itching grade at different visits [Table - 3] and [Figure - 3], which was the secondary end point for the study, shows that topical ivermectin and permethrin cause rapid improvement in itching compared to tablet ivermectin (P < 0.05). At the end of fourth week, 2 patients in permethrin group, 5 patients in oral ivermectin group, and 1 patient in topical ivermectin group failed to follow-up. They were cured clinically at the end of third week.
 |
| Figure 3: Cure rate for itching at different visits - * shows P < 0.05 for tab. ivermectin compared to permethrin cream and ivermectin lotion at the end of first and second week using Chi-square test |

Safety
No major adverse events were observed in any of the 3 groups. One patient in permethrin group developed burning sensation after applying drug, but it was relieved within few minutes. 2 patients in tablet ivermectin group developed mild headache and increase in pruritus, respectively, which subsided without any medication.
Discussion
This randomized trial was planned to evaluate the efficacy and safety of topical ivermectin and compare it with oral ivermectin and topical permethrin. Topical ivermectin as 1% lotion was as effective as 5% permethrin and was significantly more effective than oral ivermectin.
In the present study, clinical cure rate of oral ivermectin was 30% at the end of first week. Our finding differs from other studies as clinical cure rate observed at the end of first week was 79.3%, 50%, and 55.56%, respectively. [6],[8],[13]
Clinical cure rate for oral ivermectin with 2 doses was 63% at the end of second week. The cure rate was substantially lower than that reported by other studies using single dose of oral ivermectin. The cure rate reported after single dose at the end of 2 weeks were 74%, 70%, and 79%, respectively. [7],[8],[11] Bachewar et al, reported 100% cure rate with 2 doses of oral ivermectin at the end of 2 weeks. [13] While Fatimata et al, reported much lower cure rate of 24.6% at the end of second week with single dose. [12] At the end of 3 weeks, 3 doses of oral ivermectin achieved 99% cure rate. One study reported 56% cure rate with single dose at third week. [10] Several studies reported 95% cure rate with oral ivermectin at the end of 4 weeks with 2 doses of oral ivermectin. [7],[8],[11] Thus, repeating treatment every week achieves higher cure rate with oral ivermectin. However, clinical cure with topical ivermectin is seen earlier as in case of topical permethrin. Because ivermectin has not been proven to be ovicidal, a single oral dose of 200 mcg/kg body weight may be inadequate to eradicate the different stages of the parasites, and a higher dose or a second dose may be required within 1 or 2 weeks for 100% cure rate. [8]
As far as the relief from pruritus is concerned, at 3 weeks follow-up, 48% patients were free of pruritus in oral ivermectin group compared to 90.9% and 95% in permethrin and topical ivermectin group, respectively. It suggests that topical ivermectin and permethrin rapidly cured disease and hence led to rapid improvement in pruritus compared to oral ivermectin. A previous study also showed that oral ivermectin was less effective in relieving pruritus as compared to permethrin. [13] A drug with a faster effect in relieving pruritus is much more acceptable to patients. Moreover, such drug will also reduce the requirement of anti-histaminic.
While oral ivermectin was found to be significantly less effective than the 2 topical treatments, there was no difference in effectiveness of topical permethrin and topical ivermectin. This difference sustained up to 2 weeks. The faster cure with permethrin and topical ivermectin would reduce the number of follow up visits required, thus improving compliance and reducing the cost.
In all the 3 treatment groups, no severe/serious adverse events were observed. Only 3 patients had mild adverse events- mild burning sensation in permethrin group, headache, and increase in pruritus in oral ivermectin group. All these adverse events subsided without any medication. No adverse events were observed in topical ivermectin group.
A few small studies have reported efficacy of topical ivermectin in scabies. In a study of 50 patients, all the patients were cured clinically and parasitologically within 48 hours after a single application. As 50% of scabies patients had persistent itching, another application was given after 5 days to stop itching. [14] Yerham et al, reported a study of 10 patients with uncomplicated scabies, in which marked improvement was seen in the condition of the patients within 2 or 3 days of the first treatment, and clinical cure occurred after 2 or 3 days of second treatment with topical 1.8% ivermectin cream. [15] In a study consisting of 12 adults and 20 children treated with 1% ivermectin in a solution of propylene glycol applied topically to the affected skin, Victoria et al, reported that all the patients were cured with the 2 applications at weekly interval. [16] Our findings confirm these reports as single application of ivermectin achieved 69.3% cure rate and with second application, 100% cure rate was observed. This comparative randomized study shows that compared to oral ivermectin, topical ivermectin produces higher cure rate.
Limitations
The study was open-labeled. However, blinding was not easy because the formulations were different that is cream, lotion, and tablet. Possible variation, if any, due to different formulations- lotion and cream, cannot be ruled out. Still the study does show superiority of topical over oral ivermectin in uncomplicated scabies.
Conclusion
To conclude, the present study shows that the ivermectin 1% lotion is as effective as permethrin 5% cream and is definitely more effective compared to oral ivermectin in uncomplicated scabies. Topical ivermectin is also as safe as oral ivermectin and topical permethrin.
| 1. |
Scheinfeld N. Controlling scabies in institutional settings: A review of medications, treatment models, and implementation. Am J Clin Dermatol 2004;5:31-7.
[Google Scholar]
|
| 2. |
Taplin D, Meinking TL. Pyrethrins and pyrethroids in dermatology. Arch Dermatol 1990;126:213-21.
[Google Scholar]
|
| 3. |
Roos TC, Alam M, Roos S, Merk HF, Bickers DR. Pharmacotherapy of ectoparasitic infections. Drugs 2001;61:1067-88.
[Google Scholar]
|
| 4. |
Campbell WC. Ivermectin, an antiparasitic agent. Med Res Rev 1993;13:61-79.
[Google Scholar]
|
| 5. |
Glaziou P, Cartel JL, Alzieu P, Briot C, Moulia-Pelat JP, Martin PM. Comparison of ivermectin and benzyl benzoate for treatment of scabies. Trop Med Parasitol 1993;44:331-2.
[Google Scholar]
|
| 6. |
Macotela-Ruíz E, Peña-González G. The treatment of scabies with oral ivermectin. Gac Med Mex 1993;129:201-5.
[Google Scholar]
|
| 7. |
Chouela EN, Abeldaño AM, Pellerano G, La Forgia M, Papale RM, Garsd A, et al. Equivalent therapeutic efficacy and safety of ivermectin and lindane in the Treatment of Human Scabies. Arch Dermatol 1999;135:651-5.
[Google Scholar]
|
| 8. |
Usha V, Gopalakrishnan Nair TV. A comparative study of oral ivermectin and topical permethrin cream in the treatment of scabies. J Am Acad Dermatol 2000;42:236-40.
[Google Scholar]
|
| 9. |
Madan V, Jaskiran K, Gupta U, Gupta DK. Oral ivermectin in scabies patients: A comparison with 1% lindane lotion. J Dermatol 2001;28:481-4.
[Google Scholar]
|
| 10. |
Brooks PA, Grace RF. Ivermectin is better than benzyl benzoate for childhood scabies in developing countries. J Paediatr Child Health 2002;38:401-4.
[Google Scholar]
|
| 11. |
Sule HM, Thacher TD. Comparison of ivermectin and benzyl benzoate lotion for scabies in Nigerian patients. Am J Trop Med Hyg 2007;76:392-5.
[Google Scholar]
|
| 12. |
Ly F, Caumes E, Ndaw CA, Ndiaye B, Mahé A. Ivermectin versus benzyl benzoate applied once or twice to treat human scabies in Dakar, Senegal: A randomized controlled trial. Bull World Health Organ 2009;87:424-30.
[Google Scholar]
|
| 13. |
Bachewar NP, Thawani VR, Mali SN, Gharpure KJ, Shingade VP, Dakhale GN. Comparison of safety, efficacy, and cost effectiveness of benzyl benzoate, permethrin, and ivermectin in patients of scabies. Indian J Pharmacol 2009;41:9-14.
[Google Scholar]
|
| 14. |
Youssef MY, Sadaka HA, Eissa MM, el-Ariny AF. Topical application of ivermectin for human ectoparasites. Am J Trop Med Hyg 1995;53:652-3.
[Google Scholar]
|
| 15. |
Yeruham I, Hadani A. Control of human scabies by topical application of ivermectin. Ann Trop Med Parasitol 1998;92:627- 9.
[Google Scholar]
|
| 16. |
Victoria J, Trujillo R. Topical ivermectin: A new successful treatment for scabies. Pediatr Dermatol 2001;18:63-5.
[Google Scholar]
|
Fulltext Views
13,376
PDF downloads
3,141





