Translate this page into:
Acute generalized exanthematous pustulosis following intravenous immunoglobulin
Correspondence Address:
Min-Kyung Shin
Department of Dermatology, School of Medicine, Kyung Hee University, #1 Hoegi-dong, Dongdaemun-gu, Seoul 130-702
Korea
| How to cite this article: Moon SH, Shin MK. Acute generalized exanthematous pustulosis following intravenous immunoglobulin. Indian J Dermatol Venereol Leprol 2012;78:774 |
Sir,
Acute generalized exanthematous pustulosis (AGEP) is a well described but uncommon acute reaction pattern of pustular eruptions of varying etiology. The condition is most triggered by administration of systemic drugs including antibiotics, but has also been reported following viral infections. Typically, the reaction usually occurs within 48 hours following treatment with antibiotics, but may as long as 7 to 12 days to develop after treatment with other medications. [1]
A 42-year-old female with a one-year history of Guillain-Barre syndrome had been taking prednisolone for about one year. However, the lack of response to the corticosteroid treatment led to the withdrawal of prednisolone and an alternate treatment regimen consisting of 0.4 g/kg/day of intravenous immunoglobulin (IVIg). Nine days after the initiation of IVIg treatment, she developed a fever (38.5°), as well as generalized erythema and edema, and the appearance of pruritic pustular euptions over the entire body. With the exception of IVIg, there were no additional medications and no evidence of recent systemic infection. Lesions initially developed on the trunk and subsequently spread to the rest of the body. Physical examination of the patient revealed numerous erythematous, non-follicular pustules (<5 mm in diameter) on the whole body [Figure - 1] and especially severe in the folds. There was no mucosal involvement and lymphadenopathy. A routine blood test showed no abnormalities with the exception of mild neutrophilia (7200/ mm 3 ). The content of the superficial pustules was sterile.
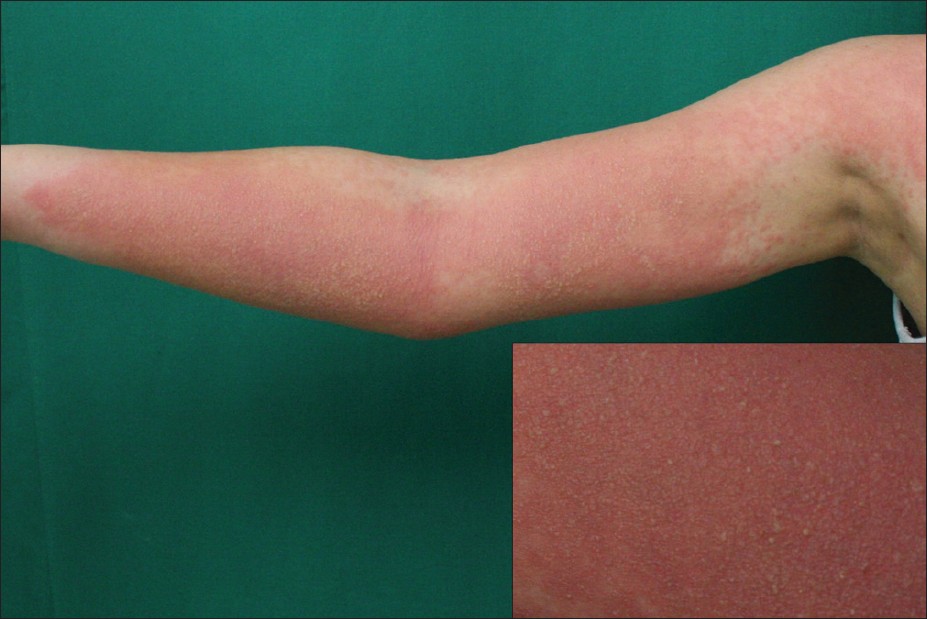 |
| Figure 1: Generalized pin-head sized, non-follicular pustules on the background of the erythema on the arm (inset: close-up of the same region showing non-follicular pustules) |
A biopsy specimen taken from a pustular lesion on the back revealed subcorneal microabcesses, spongiform intraepidermal pustules and perivascular infiltrates containing neutrophils, lymphocytes, and eosinophils in the upper dermis [Figure - 2]. A diagnosis of AGEP was suggested based on the European Study of Severe Cutaneous Adverse Reactions (Euro SCAR) score.
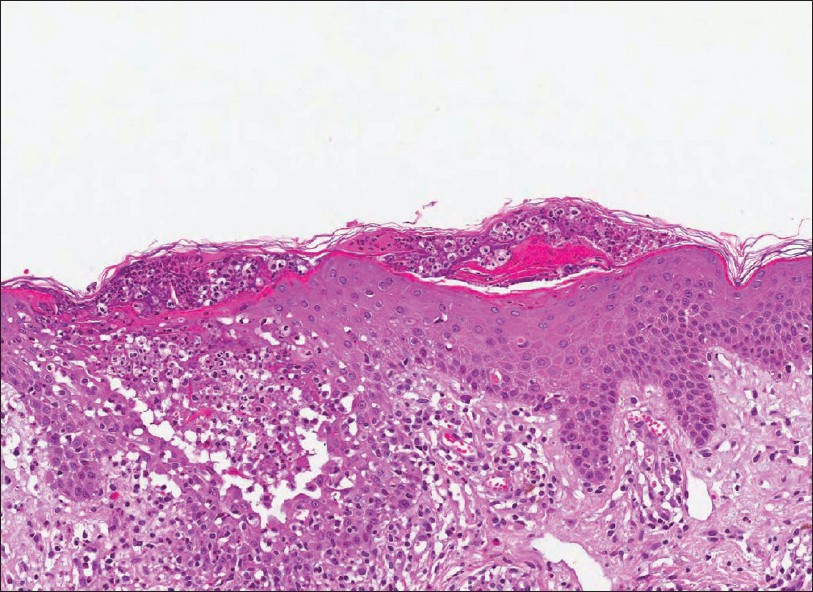 |
| Figure 2: Skin biopsy harvested from the trunk showed intra-/sub-corneal microabcess, spongiosis, intraepidermal pustules, and mixed perivascular and interstitial infiltration including eosinophils (H and E, ×200) |
The patient was treated with intravenous methyprednisolone (30 mg daily) for one week, followed by oral prednisolone with tapering for a week. After two weeks of the initiation of eruption, the skin lesion resolved completely with post-pustular pin-point desquamation. Two months after initiation of the eruption, we recommended a patch test with immunoglobulin, due to the fact that causality can be hard to prove without re-exposure. However, patch testing can occasionally be associated with further recurrence of symptoms, and the patient refused this treatment course.
The use of high-dose IVIg has become an accepted treatment for a wide spectrum of diseases, including hematologic, neurologic, connective tissue, and dermatologic diseases. [2] Adverse reactions to IVIg are usually minor and occur in about 10% of patients. [3] Adverse dermatological effects from IVIg treatment have rarely been reported and include symptoms such as urticarial or maculopapular transient rash, lichenoid cutaneous eruption, alopecia, and vasculitis. [3] As reported in our case study, the cutaneous eruptions usually occur after following the first IVIg infusion (82% of reported cases [4] ).
However, the pathophysiology and natural course are unknown. A hypersensitivity reaction to a specific ingredient in the IVIg is possible; however, patch and prick tests with immunoglobulins and excipients have always been negative as previously reported. Our hypothesis of induced AGEP following IVIg treatment is based on very high levels of IgG (4540 mg/dL) prior to IVIg administration. The patient IgG could have been a much higher level because immunoglobulins consist of more than 90% of IgG. Therefore, temporary increased levels of IgG may induce antigen mimicry within keratinocytes. [5]
To the best of our knowledge, this is the first report of a case of AGEP that was probably induced by IVIg. Eczematous eruptions due to the infusion of immunoglobulins are known to be rare and cause mostly benign side effects. However, high-dose intravenous immunoglobulins have emerged as an important therapy for various diseases including those with a dermatological component. Further study is required to determine the relevance of the IVIg-induced AGEP development and its interaction with various disorders.
| 1. |
Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). Br J Dermatol 2007;157:989-96.
[Google Scholar]
|
| 2. |
Mittmann N, Chan B, Knowles S, Cosentino L, Shear N. Intravenous immunoglobulin use in patients with toxic epidermal necrolysis and Stevens-Johnson syndrome. Am J Clin Dermatol 2006;7:359-68.
[Google Scholar]
|
| 3. |
Nydegger UE, Sturzenegger M. Adverse effects of intravenous immunoglobulin therapy. Drug Safety 1999;21:171-85.
[Google Scholar]
|
| 4. |
Cohen Aubart F, Barete S, Amoura Z, Francès C, Lyon-Caen O, Lebrun-Vignes B. Intravenous immunoglobulins-induced eczematous eruption: A long-term follow-up study. Eur J Intern Med 2009;20:70-3.
[Google Scholar]
|
| 5. |
Ikeda K, Iwasaki Y, Ichikawa Y, Kinoshia M. Pompholyx after IV immunoglobulin therapy for neurologic disease. Neurology 2000;54:1879.
[Google Scholar]
|
Fulltext Views
2,920
PDF downloads
3,247





