Translate this page into:
Cutaneous solar ultraviolet exposure and clinical aspects of photodamage
Correspondence Address:
Claire Battie
5-29 quai Aulagnier - 92665 Asnieres Sur Seine
France
| How to cite this article: Battie C, Verschoore M. Cutaneous solar ultraviolet exposure and clinical aspects of photodamage. Indian J Dermatol Venereol Leprol 2012;78:9-14 |
Abstract
Solar ultraviolet (UV) radiation reaching the earth is a combination of UVB (290-320 nm) and UVA (320-400 nm) wavelengths. Since UVA is less energetic than UVB, UVB has long been thought to be the factor responsible for the damaging effects of solar radiation. But with modern tools such as in vitro models, it has been proven that UVA plays a major role. The objective of this review is to show how skin may be exposed to UV light and to highlight the clinical aspects of UV-induced skin damages with the respective contribution of UVB or UVA. Even if UVA is less energetic than UVB, it is more abundant and penetrates deeper into the skin, reaching as far as the dermis. Various factors also influence skin exposure to UV light: the latitude, season, and time of the day. Acute as well as chronic sun exposure induces short- and long-term clinical damages. Erythema and pigmentation are immediate responses of normal human skin exposed to UV radiation. The long-term effects are photoaging and photocarcinogenesis. In particular, UVA appears to play a major role in the deterioration of dermal structure leading to the photoaged appearance of the skin.Cutaneous UV Exposure
Solar radiation reaching the skin
The solar spectrum includes several wavebands ranging from the very short cosmic rays to very long radio waves and beyond. Solar radiation reaching the surface of the earth, and thereby the surface of our skin, contains infrared (700-2500 nm), visible (400-700 nm), and ultraviolet radiation (UVR) (290-400 nm). UVR is invisible.
Although UVR represents less than 9% of the total solar irradiance between 290 and 2500 nm received on the earth′s surface, [1] the UV photons have the greatest biological impact. More precisely, there are three categories of UVR. UVC rays (100-290 nm) are the shortest in wavelength and are filtered out by the ozone layer. In contrast, UVB rays (290-320 nm) and UVA (320-400 nm) reach the earth′s surface and are responsible for cutaneous photobiological events. UVA can be further subdivided into longer wavelengths, UVA1 (340-400 nm), and shorter wavelengths, UVA2 (320-340 nm). [2]
UVB radiation reaches the earth in relatively low amounts (about 0.5% of solar spectral irradiance at ground level, integrated over 290-2500 nm range) and is highly energetic. In contrast, UVA rays are lower in energy, but they are at least 20 times more abundant. 95% of UV rays reaching the ground level are UVA. [1]
Various factors influencing skin exposure to solar ultraviolet rays
The solar UV irradiance highly varies because it depends on geo-orbital and environmental parameters.
Geo-orbital parameters include latitude, date of the year, and hour of the day. [3],[4] All these factors are related to the height of the sun in the sky, and hence the pathway of beam of sunlight through the atmosphere. Because of the elliptical orbit of the earth around the sun, the distance between the sun and the earth varies by about 3.4% over the year. This results in a variation of about 7% in intensity and in slightly higher levels of UVR in summer in the southern than in the northern hemisphere. Both the quality (spectrum) and the quantity (intensity) of terrestrial UVR vary with sun′s elevation above the horizon, or solar altitude. The solar altitude depends on the time of the day, day of the year, and geographic location (latitude and longitude). On a summer day, the UV energy received (daily dose) includes approximately 3.5% UVB and 96.5% UVA. [5] UV irradiance is greater for both UVA and UVB with decreasing latitude. [3]
[Figure - 1] shows the variation of UVB and UVA irradiance during a clear summer day in South of France. Balasaraswathy et al. also showed that both the UVA and UVB reached a peak between 11.30 a.m. and 1.30 p.m. in Coimbatore, India. [6] In addition, this study highlighted the fact that UVB radiation was much lower than UVA radiation in the morning and in the evening.
 |
| Figure 1: Variations of UVB (blue line) and UVA (red line) irradiances along a clear summer day in south of France |
The dose of UVR reaching the skin also depends on the season. UVB irradiance is much higher in summer than in winter at a given site. UVA irradiance is less affected by seasons and decreases to a lesser extent in winter. [3] In Coimbatore, compared to average irradiance between March and October, UVB was lower in November, December, January, and February by 24%, 40%, 19%, and 12%, respectively, and UVA was lower by 13%, 22%, 18%, and 13%, respectively. [6]
Environmental parameters can also influence UV exposure. They include the ozone total column and the ozone vertical atmospheric profile, clouds, pollutants, dusts, aerosols, and albedo (reflection of UVR from the ground). [7] Absorption by ozone, in addition to cutting off UVC radiation, has a dramatic influence on the amount of UVB radiation reaching the ground. [8],[9],[10],[11],[12],[13] As aerosols and dusts are less concentrated at high altitudes, UV irradiance values are higher in a mountain location than at the sea level. Finally, a highly reflecting environment, such as white sand, fresh snow or, to a lesser extent, white broken clouds acting as reflectors, can significantly increase UV irradiance. [14]
Furthermore, the main part of UVA radiation is not absorbed by standard glass: car windows, verandas, conservatories, and windows in general fail to protect against UVA as they do from UVB radiation because the glass short cut-off wavelength is about 320 nm. Thus, high UVA doses may be received while erythemal UVB is filtered out.
The contribution of diffuse UVR is also important and should not be underestimated. Indeed, a recent study suggests that diffuse irradiation may explain a large part of the cumulative annual exposure dose. [15]
Solar ultraviolet penetration throughout the skin
70% of UVB radiation that reaches the skin is absorbed by the stratum corneum, 20% reaches viable epidermis, and only 10% penetrates the upper part of the dermis. On the other hand, UVA radiation is partly absorbed by the epidermis, but 20-30% of it reaches deep dermis. Thus, UVA rays are more penetrating than UVB ones. The major chromophores that determine the depth of penetration are nucleic acids, aromatic amino acids, and melanin. So, UVB has a major action on the epidermis and UVA can also target the dermis [Figure - 2].
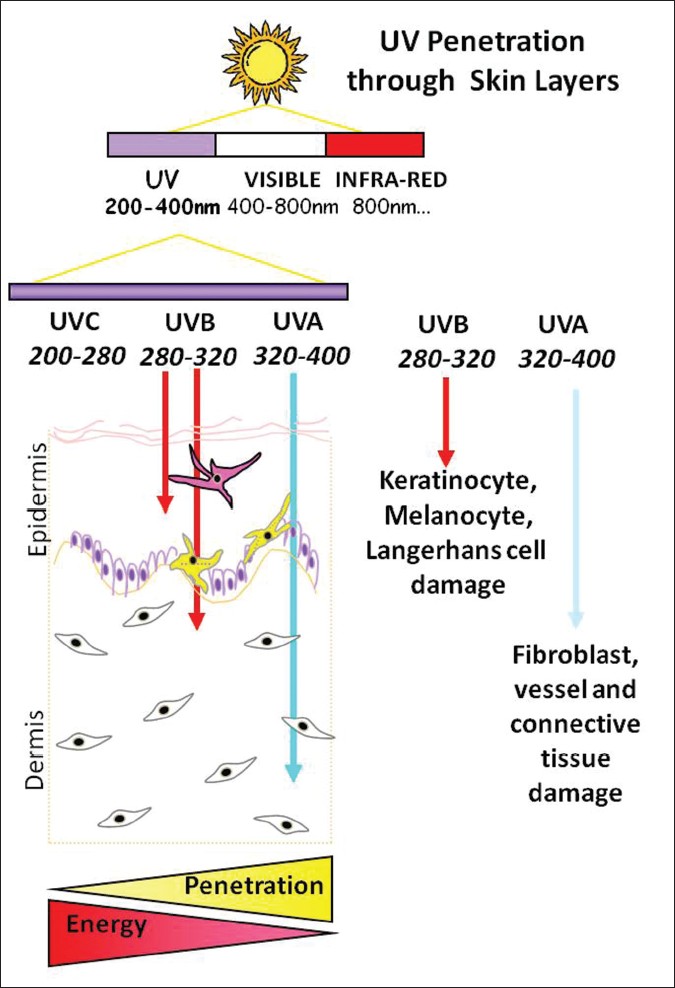 |
| Figure 2: Diagram showing depth of UV penetration into the skin and photon-associated energy according to wavelength: UVA penetrates deeper |
Clinical Aspects of UV-Induced Skin Damage
Short-term effects
Sunburn (erythema) and suntan (pigmentation) are the immediate responses of normal human skin exposed to UVR.
Erythema
Erythema (sunburn) is the most familiar symptom associated with UVR overexposure. It is an acute skin inflammatory reaction associated with redness. The erythemal reaction to UVR depends on the wavelength range. Increasing wavelength decreases considerably the erythemal effectiveness. UVB, particularly at 307 nm, is the most effective waveband for eliciting erythema in the human skin. UVA radiation is 1000-fold less potent in producing skin erythema.
UVB-induced erythema is a delayed response. It reaches a peak at 6-24 h depending on the dose, [16] with erythema, pruritus, and pain in sun-exposed areas. This erythema fades over a day or longer, depending on the dose and the skin type. [17] In skin type I, it may last longer compared to skin type III or IV. [4] UVA-induced erythema contributes to at least 15% of total sun-induced erythema. [18] The minimal erythema dose (MED) is defined as the UVB dose that induces minimally perceptible or detectable erythema. This biological value obviously varies from one subject to another. It depends on the skin phototype as well as the skin color typing and body area. MED increases with higher skin type. [19] Since most Indians have Fitzpatrick skin phototypes III-V, they obviously have a higher MED than Caucasian skin. It is nevertheless important to note that there is a considerable overlap of MED between skin phototypes, especially in the mid-dose range [Figure - 3]. Similarly, people involved in outdoor occupation have a higher MED as compared to people involved in indoor occupation.
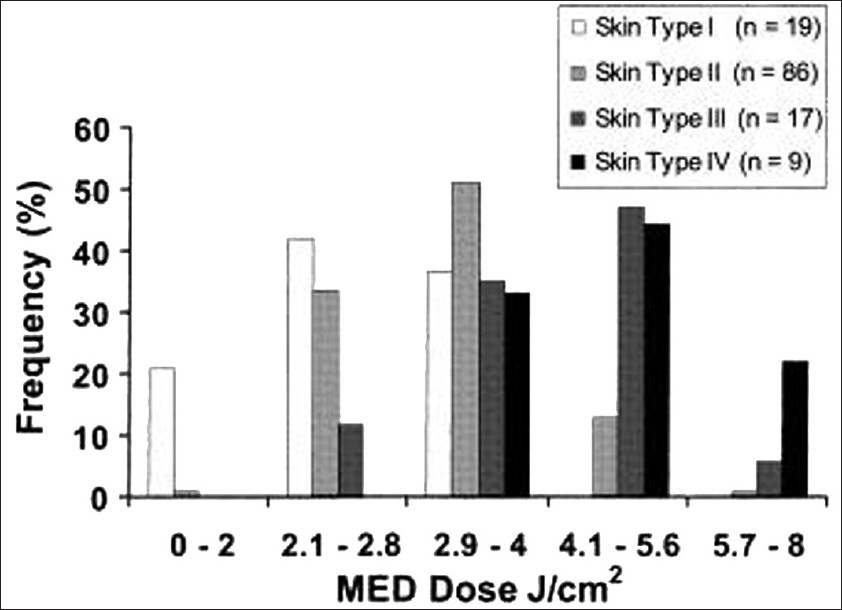 |
| Figure 3: Distribution of MED with SSR filter in skin types I-IV. These data show a considerable overlap, especially in the mid-dose range (from 19) |
Later changes include hyperkeratosis (increased scaling), acanthosis (epidermal thickening), disorganization and misalignment of keratinocytes, dermal vascular ectasia, and mononuclear perivascular infiltration.
Pigmentation
Sun exposure induces the UVA and UVB pigmentation phenomena. UVA-induced changes in color begin with an immediate darkening of the skin due to photo-oxidation of pre-existing melanin [immediate pigment darkening (IPD)]. [18],[20] In skin types III and IV, this pigmentation may appear within a short single exposure to UVA (dose less than 6 J/cm). [21] A partial fading occurs rapidly within 1 h after the end of exposure. As it decreases, the pigmentation progressively loses its blue component within 2 h post-exposure. The phenomenon is more prominent in darkly pigmented individuals and it does not protect the skin against the effects of UVB radiation. [4]
Following exposure to UVA doses higher than about 10 J/cm 2 , a stable residual pigmentation is observed after the transient part of IPD has faded out. This pigmentation [persistent pigment darkening (PPD)] remains detectable for a few days or weeks, depending on the UVA dose applied and this is particularly seen in skin with phototypes III or IV. [21] It is also due to melanin photo-oxidation. A minimal PPD dose is about 15 J/cm 2 and represents somewhat less than the UVA dose received over 1 h of exposure to a quasi-zenithal sun. [22]
The neo-melanization or delayed pigmentation is characterized by a visible brown pigmentation in UV-exposed skin, which represents an increase in epidermal melanin content. It becomes visible after about 72 h. An acute erythemogenic dose of UVB is necessary to induce delayed pigmentation. Both UVA and UVB can cause tanning, but UVA is less effective. However, melanization produced by cumulative UVA exposures appears to be much longer lasting (several months or even a year) than that acquired with UVB exposures.
UVB pigmentation phenomena result in a homogeneous color, which can bring some natural protection. On the contrary, UVA pigmentation is not protective, as shown by the absorbance spectra of UVA-induced pigmentation which is under 0 from 290 to 400 nm. [23]
UV-pigmentation can lead to irregular pigmentation and hyperpigmented areas. In particular, melasma [Figure - 4], post-inflammatory pigmentation, and actinic lentigines are associated with exposure to UVR. [24]
 |
| Figure 4: Melasma of the face in an Indian man (Courtesy: Prof. Ortonne) |
Pigmented changes are the major sign of skin photoaging in Asians. [25],[26],[27] An ethnic group-related variation in melanosome distribution was reported, [28],[29],[30],[31],[32] showing a mix of individual (about 60%) and aggregated (about 40%) melanosomes in Asian skin, whereas aggregated melanosomes (85%) prevail in European skin. [32],[33] The density and highly variable size of melanosomes in Asian skin could account for the irregular, spotty pigmentation associated with photoaging. It is also known that in darker-skinned individuals, UVA induces greater pigmenting effects than UVB. [34]
Long-term effects: Photoaging and photocarcinogenesis
Photoaging
The damage caused to the skin by chronic sun exposure differs in many respects from natural aging. Photoaged skin is characterized by numerous clinical signs, fine and coarse wrinkling, laxity, leathery appearance, mottled pigmentation reflected by lentigines, fragility, impaired wound healing, and telangiectasias. [Figure - 5] clearly illustrates this impact of sun exposure on skin.
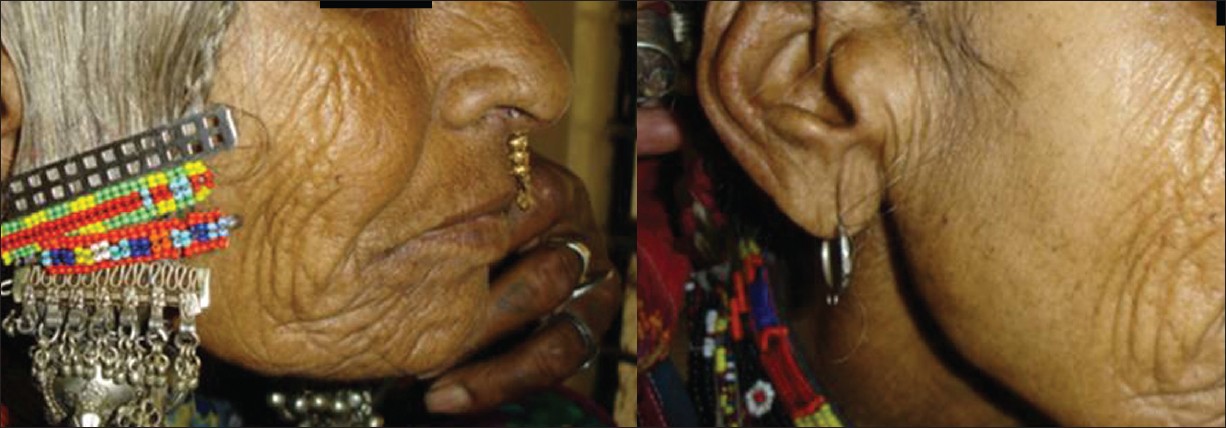 |
| Figure 5: A 70-year-old Indian woman -Sun-protected versus sun-exposed skin (Courtesy: Prof. Inamadar) |
Histologic and ultrastructural studies have revealed that the major alterations in photoaged skin are found in the connective tissue (dermis). [35],[36],[37] Damage induced by UVR is primarily reflected by an impaired collagen fibril network and accumulation of abnormal, amorphous, elastin-containing material. [38] Increased lysozyme staining on abnormal elastic fibers from sun-damaged skin has been reported. [39] As lysozyme at high concentrations inhibits the activity of collagenase and elastase, it prevents the elastic fibers component from proteolysis. Greater deposition follows repeated UVA exposure. In actinically damaged skin, there is also a loss of collagen associated with change in collagen composition (i.e. an increase in collagen III/collagen I ratio). There is a significant correlation between reduced level of type I collagen and the severity of photodamage in human skin. [40]
Since collagen fibrils and elastin are responsible for the firmness and resilience of skin, their disarrangement induced by photoaging process causes the skin to look older. [41],[42]
While the roles of UVB and UVA wavelengths in the photoaging process are not fully understood, it is known that UVA radiation contributes significantly to long-term deterioration of the dermal structure and clinical signs of photoaging. [43] In particular, repeated exposures to UVA induce alterations within the dermal compartment, which correlate with early damage occurring during photoaging. [44] An in vivo study showed that using repeated low doses of solar simulated radiation (SSR) for 6 weeks induces the production of some of the major alterations observed and/or participating in the long-term photoaging process (e.g. reduced level of type I collagen precursor, increased lysozyme deposit on elastic fibers). This study also demonstrated the efficacy of a daily broad-spectrum photoprotection in preventing some of those biological endpoints. [45]
Photocarcinogenesis
Sunlight overexposure is involved in increasing the risk of skin cancer since DNA represents one of its biological targets. Indeed, DNA alteration can affect many cellular functions and can lead to mutations and genetic instability. Unlike UVB which directly impacts DNA, UVA toxicity mainly depends on indirect mechanisms in which reactive oxygen species (ROS) are generated through the activation of endogenous photosensitizers present in skin, triggering the genotoxic effects. Thus, repetitive low-dose UVA is capable of eliciting DNA damage. Evidence for the generation of oxidative damage in cultured cells, and even in skin biopsy specimens, has been accumulating in recent years; several reports have described the induction of transient DNA breakage after UVA exposure. Purines and pyrimidines can be modified by ROS. One of the best studied lesions is 8 oxo-dG, which results from the oxidation of the guanine moiety. This 8 oxo-dG lesion was shown to be premutagenic and it is suspected to be involved in the photocarcinogenic process initiated by sunlight. [46]
Regarding the clinical data, there is strong evidence to support the direct role of sunlight exposure in the development of skin cancers, especially non-melanoma skin cancers (NMSCs), squamous cell carcinoma (SCC), and basal cell carcinoma (BCC). [47] These cancers occur more frequently on the head, neck, arms, and hands, which are the skin areas most frequently exposed to UVR. Actinic keratoses (AK), which are precancerous lesions, are also frequent in these body sites.About 5-20% of these lesions progress to SCC. Lightly pigmented individuals (skin types I or II) are more prone to NMSC than those with deeply pigmented skin. [48] Conventional wisdom has it that the incidence of all varieties of skin cancers is lower among Indians due to the protective effects of melanin. Nevertheless, a recent Indian review showed that there are indirect indications that NMSCs may be on the rise in India. [49]
Unlike NMSC, the direct association with UV exposure is still under investigation for cutaneous malignant melanoma. Severe sunburn episodes during childhood may cause the development of melanoma on sun-exposed areas. A recent Australian study tends to prove that melanoma may be preventable by regular sunscreen use in adults. [50]
| 1. |
Commission Internationale de l'Éclairage, 1989. Publ. CIE N° 85 Available from: http://www.cie.co.at/index.php/Publications/index.php?i_ca_id=360. [Last accessed 2011 Jan 20].
[Google Scholar]
|
| 2. |
Moyal D, Fourtanier A. Acute and Chronic Effects of UV on skin: What are they and how to study them? In: Rigel DS, Weiss RA, Lim HW, Dover JS, editors. Photoaging. New York: Marcel Dekker, Inc; 2004. p. 15-32.
[Google Scholar]
|
| 3. |
Sabziparvar AA, Shine KP, Forster PM. A model-derived global climatology of UV irradiation at the Earth's surface. Photochem Photobiol 1999;69:193-202.
[Google Scholar]
|
| 4. |
Kollias N, Malallah YH, al-Ajmi H, Baqer A, Johnson BE, González S. Erythema and melanogenesis action spectra in heavily pigmented individuals as compared to fair-skinned Caucasians. Photodermatol Photoimmunol Photomed 1996;12:183-8.
[Google Scholar]
|
| 5. |
Diffey BL. What is light? Photodermatol Photoimmunol Photomed 2002;18:68-74.
[Google Scholar]
|
| 6. |
Balasaraswathy P, Kumar U, Srinivas CR, Nair S. UVA and UVB in sunlight, Optimal Utilization of UV rays in Sunlight for phototherapy. Indian J Dermatol Venereol Leprol 2002;68:198-201.
[Google Scholar]
|
| 7. |
Christiaens FJ, Chardon A, Fourtanier A, Frederick JE. Standard ultraviolet daylight for nonextreme exposure conditions. Photochem Photobiol 2005;81:874-8.
[Google Scholar]
|
| 8. |
Frederick JE, Lubin D. Possible long-term changes in biologically active ultraviolet radiation reaching the ground. Photochem Photobiol 1988;47:571-8.
[Google Scholar]
|
| 9. |
Frederick JE, Snell EH, Haywood EK. Solar ultraviolet radiation at the Earth's surface. Photochem Photobiol 1989;50:443-50.
[Google Scholar]
|
| 10. |
Lubin D, Jensen EH. Effects of clouds and stratospheric ozone depletion on ultraviolet radiation trends. Nature 1995;377:710-3.
[Google Scholar]
|
| 11. |
Ma J, Guicherit R. Effects of stratospheric ozone depletion and tropospheric pollution on UVB radiation in the troposphere. Photochem Photobiol 1997;66:346-55.
[Google Scholar]
|
| 12. |
Loughlin PE, Box MA. Investigating biological response in the UVB as a function of ozone variation using perturbation theory. J Photochem Photobiol B 1998;43:73-85.
[Google Scholar]
|
| 13. |
Krzyscin JW. Total ozone influence on the surface UV-B radiation in the late spring-summer 1963-1997: An analysis of multiple timescales. J Geophys Res 2000;105:4993-5000.
[Google Scholar]
|
| 14. |
Frederick JE, Qu Z, Booth CR. Ultraviolet radiation at sites on the Antarctic coast. Photochem Photobiol 1998;68:183-90.
[Google Scholar]
|
| 15. |
Vernez D, Milon A, Vuilleumier L, Bulliard JL. Anatomical exposure patterns of skin to sunlight: Relative contributions of direct, diffuse and reflected ultraviolet radiation. Br J Dermatol 2012 [Epub ahead of print]
[Google Scholar]
|
| 16. |
Farr PM, Diffey BL. The erythemal response of human skin to ultraviolet radiation. Br J Dermatol 1985;113:65-76.
[Google Scholar]
|
| 17. |
Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 1988;124:869-71.
[Google Scholar]
|
| 18. |
Kaidbey KH, Kligman AM. The acute effects of long-wave ultraviolet radiation on human skin. J Invest Dermatol 1979;72:253-6.
[Google Scholar]
|
| 19. |
Harrison GI, Young AR. Ultraviolet radiation-induced erythema in human skin. Methods 2002;28:14-9.
[Google Scholar]
|
| 20. |
Routaboul C, Denis A, Vinche A. Immediate pigment darkening: Description, kinetic and biological function. Eur J Dermatol 1999;9:95-9.
[Google Scholar]
|
| 21. |
Chardon A, Moyal D, Hourseau C. Persistent pigment darkening response as a method for evaluation of UVA protection assays. In: Lowe NJ, Shath NA, Pathak MA, editors. Sunscreens: Development, Evaluation and Regulatory Aspects. 2 nd ed. New York: Marcel Dekker; 1996. p. 559-82.
[Google Scholar]
|
| 22. |
Moyal D, Chardon A, Kollias N. Determination of UVA protection factors using the persistent pigment darkening (PPD) as the end point. (Part 1). Calibration of the method. Photodermatol Photoimmunol Photomed 2000;16:245-9.
[Google Scholar]
|
| 23. |
Young AR, Sheehan JM. UV-induced pigmentation in human skin. In: Giacomoni PU, editor. Sun Protection in Man. Vol. 3. Amsterdam: Elsevier Science; 2002. p. 357-75.
[Google Scholar]
|
| 24. |
Khanna N, Rasool S. Facial melanoses: Indian perspective. Indian J Dermatol Venereol Leprol 2011;77:552-63.
[Google Scholar]
|
| 25. |
Zhao P, Zhu X, Liu Y, Wang B, Wang C, Burns FJ. Solar ultraviolet radiation and skin damage: An epidemiological study among a Chinese population. Arch Environ Health 1998;53:405-9.
[Google Scholar]
|
| 26. |
Chung JH. Photoaging in Asians. Photodermatol Photoimmunol Photomed 2003;19:109-21.
[Google Scholar]
|
| 27. |
Goh SH. The treatment of visible signs of senescence: The Asian experience. Br J Dermatol 1990;122:105-9.
[Google Scholar]
|
| 28. |
Alaluf S, Atkins D, Barrett K, Blount M, Carter N, Heath A. Ethnic variation in melanin content and composition in photoexposed and photoprotected human skin. Pigment Cell Res 2002;15:112-8.
[Google Scholar]
|
| 29. |
Szabo G, Gerald AB, Pathak MA, Fitzpatrick TB. Racial differences in the fate of melanosomes in human epidermis. Nature 1969;222:1081-92.
[Google Scholar]
|
| 30. |
Toda K, Pathak MA, Parrish JA, Fitzpatrick TB, Quevedo WC. Alteration of racial differences in melanosome distribution in human epidermis after exposure to ultraviolet light. Nat New Biol 1972;236:143-5.
[Google Scholar]
|
| 31. |
Konrad K, Wolff K. Hyperpigmentation, melanosome size, and distribution patterns of melanosomes. Arch Dermatol 1973;107:853-60.
[Google Scholar]
|
| 32. |
Thong HY, Jee SH, Sun CC, Boissy RE. The patterns of melanosome distribution in keratinocytes of human skin as one determining factor of skin colour. Br J Dermatol 2003;149:498-505.
[Google Scholar]
|
| 33. |
Jimbow M, Jimbow K. Pigmentary disorders in oriental skin. Clin Dermatol 1989;7:11-27.
[Google Scholar]
|
| 34. |
Kollias N, Malallah YH, al-Ajmi H, Baqer A, Johnson BE, González S. Erythema and melanogenesis action spectra in heavily pigmented individuals as compared to fair-skinned Caucasians. Photodermatol Photoimmunol Photomed 1996;12:183-8.
[Google Scholar]
|
| 35. |
Tsoureli-Nikita E, Watson RE, Griffiths CE. Photoageing: The darker side of the sun. Photochem Photobiol Sci 2006;5:160-4.
[Google Scholar]
|
| 36. |
Griffiths CE. The clinical identification and quantification of photodamage. Br J Dermatol 1992;127:37-42.
[Google Scholar]
|
| 37. |
Smith JG, Davidson EA, Sams WM Jr, Clark RD. Alterations in human dermal connective tissue with age and chronic sun damage. J Invest Dermatol 1962;39:347-50.
[Google Scholar]
|
| 38. |
Lavker RM, Veres DA, Irwin CJ, Kaidbey KH. Quantitative assessment of cumulative damage from repetitive exposures to suberythemogenic doses of UVA in human skin. Photochem Photobiol 1995;62:348-52.
[Google Scholar]
|
| 39. |
Seite S, Zucchi H, Septier D, Igondjo-Tchen S, Senni K, Godeau G. Elastin changes during chronological and photo-ageing: The important role of lysozyme. J Eur Acad Dermatol Venereol 2006;20:980-7.
[Google Scholar]
|
| 40. |
Warren R, Gartstein V, Kligman AM, Montagna W, Allendorf RA, Ridder GM. Age, sunlight, and facial skin: A histologic and quantitative study. J Am Acad Dermatol 1991;25:751-60.
[Google Scholar]
|
| 41. |
Seité S, Moyal D, Richard S, de Rigal J, Lévêque JL, Hourseau C, et al. Mexoryl SX, a broad spectrum UVA filter protects human skin from the effects of repeated suberythemal doses of UVA. J Photochem Photobiol B 1998;44:69-76.
[Google Scholar]
|
| 42. |
Fourtanier AF, Bernerd F, Bouillon C, Marrot L, Moyal D, Seite S. Protection of skin biological targets by different types of sunscreens. Photodermatol Photoimmunol Photomed 2006;22:22-32.
[Google Scholar]
|
| 43. |
Lavker RM, Gerberick GF, Veres D, Irwin CJ, Kaidbey KH. Cumulative effects from repeated exposures to suberythemal doses of UVB and UVA in human skin. J Am Acad Dermatol 1995;32:53-62.
[Google Scholar]
|
| 44. |
Seite S, Moyal D, Richard S, de Rigal J, Leveque JL. Effects of repeated suberythemal doses of UVA in human skin. In: Rougier A, Schaefer H, editors. Protection of the Skin Against Ultraviolet Radiations. Paris: John Libbey Eurotext; 1998. p. 159-66.
[Google Scholar]
|
| 45. |
Seité S, Fourtanier A. The benefit of daily photoprotection. J Am Acad Dermatol 2008;58:S160-6.
[Google Scholar]
|
| 46. |
Marrot L, Meunier JR. Skin DNA photodamage and its biological consequences. J Am Acad Dermatol 2008;58:S139-48.
[Google Scholar]
|
| 47. |
Black HS, de Gruijl FR, Forbes PD, Cleaver JE, Ananthaswamy HN, De Fabo EC, et al. Photocarcinogenesis: An overview. J Photochem Photobiol B 1997;40:29-47.
[Google Scholar]
|
| 48. |
Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer 1995;75:667-73.
[Google Scholar]
|
| 49. |
Panda S. Nonmelanoma skin cancer in India: Current scenario. Indian J Dermatol 2010;55:373-8.
[Google Scholar]
|
| 50. |
Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: Randomized trial follow-up. J Clin Oncol 2011;29:257-63.
[Google Scholar]
|
Fulltext Views
13,937
PDF downloads
2,759





