Translate this page into:
Two uncommon cases of idiopathic atrophoderma of pasini and pierini: Multiple and giant
2 Department of Dermatology, First Affiliated Hospital, Nanjing Medical University, Nanjing, China
Correspondence Address:
Zhu Wen-Yuan
Department of Dermatology, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing 210029
China
| How to cite this article: Ru-Zhi Z, Wen-Yuan Z. Two uncommon cases of idiopathic atrophoderma of pasini and pierini: Multiple and giant. Indian J Dermatol Venereol Leprol 2011;77:402 |
Abstract
We report a 39-year-old Chinese man presenting with approximately 200 atrophic brownish patches for 10 years, whose clinical manifestation and pathological features were consistent with the findings of idiopathic atrophoderma of Pasini and Pierini. Another described case was a 62-year-old woman who had a gradually increasing asymptomatic hyperpigmented and depressed patch on her right lumbosacral region over a period of 7 years. At the time of consultation, the size of the lesion was approximately 27 Χ 23 cm.Introduction
Idiopathic atrophoderma of Pasini-Pierini (IAPP) is an uncommon dermatologic condition of unknown etiology, characterized by asymptomatic, violaceous brownish, discolored patch, usually presenting as one or more sharply demarcated depressed lesions. The primary cutaneous atrophy first described by Pasini and Pierini had attracted little attention until a detailed description was given by Canizares et al. [1] who first introduced this disorder into the American dermatologic literature and proposed the term IAPP. The lesions are usually round or ovoid, varying in size from few millimeters to several centimeters, and are often oriented along the cleavage lines and possibly coalescing to form large, irregular-shaped plaques with convex borders. [1] Herein, we present two uncommon cases, namely, multiple and giant IAPP, respectively.
Case Reports
Case 1
A 39-year-old Chinese man presented with a 10-year history of multiple atrophic brownish patches on his shoulders, back, and lumbar parts. He had neither prior trauma nor tick bites at those sites. His medical and familial histories were unremarkable. The patient′s general health was excellent. Laboratory findings, including a complete blood count, liver function test, urinalysis, and electrolytes were within normal limits. Antinuclear antibodies, rheumatoid factor, anti-dsDNA antibody and anti-Scl70 antibodies were negative. Serum antibodies for Borrelia burgdorferi were negative.
Physical examination showed over 200 depressed dark brown plaques with "cliff-drop" border scattered on his back, both shoulders, and lumbar region. They ranged from few millimeters to several centimeters in size and were oriented along the cleavage lines [Figure - 1]. They tended to be homogeneous in color with hues varying from dark red to brown [Figure - 2]. These lesions looked like "footprints in the snow" or "Swiss cheese." No inflammatory component was present.
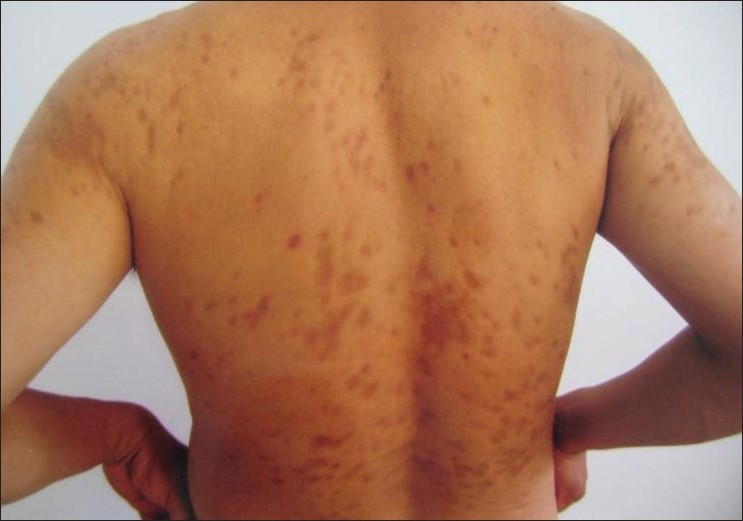 |
| Figure 1: Multiple atrophic erythematous plaques on the back, shoulders, and the extensor aspects of the proximal ends of upper limbs |
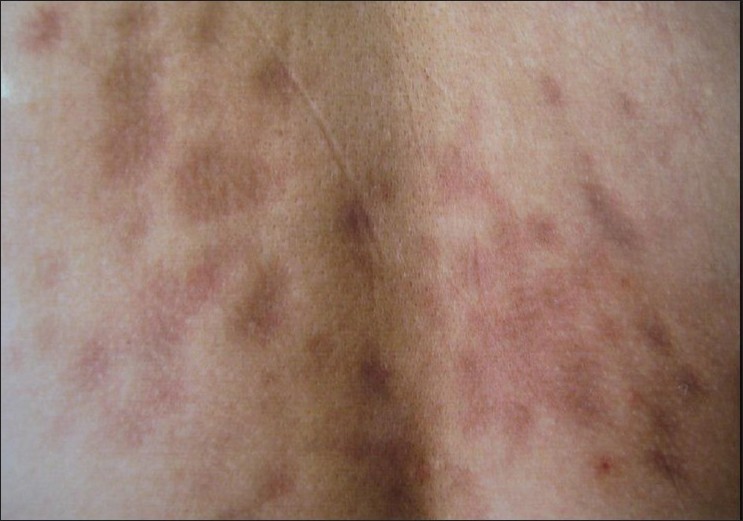 |
| Figure 2: Close view of Figure 1. Purplish and slightly depressed macular lesions on the dorsocentral region |
A skin biopsy taken from the atrophic pigmented lesion on the back revealed an increased amount of melanin in the basal cell layer and thickened, tightly packed collagen bundles in the dermis. In the atrophic lesion, slightly atrophic epidermis and thinner dermis were observed as compared with the adjacent normal skin. Neither inflammatory cells nor vascular changes were seen in the dermis [Figure - 3]. The final diagnosis was made and the patient was comforted by the fact that this disease usually follows a benign course. Danshen pill, which is a traditional Chinese medicine, whose pharmacological action is mainly activating blood circulation to dissipate blood stasis, was prescribed to be taken orally.
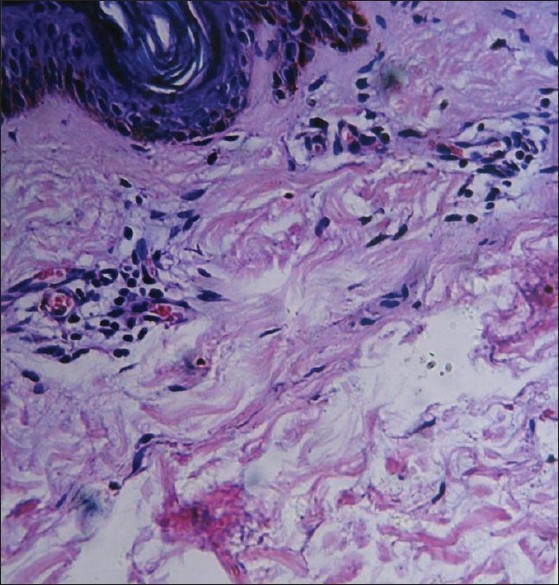 |
| Figure 3: The involved skin showed atrophic epidermis, an increased amount of melanin in the basal cell layer, and thickened, tightly packed collagen bundles in the dermis (H and E, ×200) |
Case 2
A 62-year-old woman was referred to us because of the onset and progress, over a period of 7 years, of a giant asymptomatic hyperpigmented and depressed patch on her right lumbosacral region. The patient did not report preceding redness, induration, or a history of trauma. The patient had a history of hypothyroidism, Sjogren syndrome, and urticarial vasculitis. She persistently took one pellet of tetraiodothyronine once a day, for treating hypothyroidism. The traditional Chinese medicine was administered off and on for controlling the symptoms of Sjogren syndrome.
On examination, a single, slightly depressed and hyperpigmented brownish patch with normal texture, approximately of size 27 Χ 23 cm, was present on the right back and lumbosacral region [Figure - 4]. The macule was irregularly shaped with ill-defined borders. No elevated active red border was noticed. Superficial veins could be clearly seen in the region, especially in the central zone. No signs of hardening or inflammation were found. The lesions were nonindurated except for two areas, which were of ivory and dull red color, in the back and the low right flank, in the sizes of 4 Χ 3 cm and 0.5 Χ 2 cm, respectively, and clinically compatible with morphea.
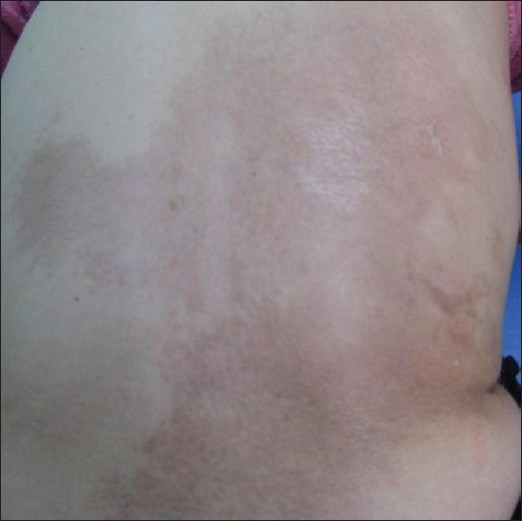 |
| Figure 4: A single slightly depressed mauve patch with normal texture, approximately of a size 27 × 23 cm, was present on the right back and lumbosacral region |
In the lower right region of the brown patch, there was an irregular, dull red plaque with infiltrative at palpation. Her face, hands, and feet were spared. No stigmata of scleroderma were apparent, and the rest of the physical examination was unremarkable. Histopathology of the skin lesions could not be performed as the patient refused a skin biopsy.
Laboratory investigations revealed normal full blood count, erythrocyte sedimentation rate, urea, electrolytes, glucose, liver function tests, serum protein electrophoresis, and negative autoantibody screen.
Although our request for biopsy was refused, the diagnosis of APP was made in consideration of the clinical examination. Considering the fact that the patient had taken some drugs including hydroxychloroquine orally for the treatment of hypothyroidism, Sjogren syndrome, and urticarial vasculitis, only external application of heparin sodium cream twice daily was prescribed.
Discussion
The cause and etiopathogenesis of IAPP remains elusive. Previous studies suggested that genetic factors, neurogenic cause, immunological factors, and abnormal metabolism of dermatan sulfate may play a role in the pathogenesis of IAPP. [2],[3],[4],[5] The role of Borrelia burgdorferi remains controversial. [6] IAPP is classically thought of as an idiopathic atrophy of the dermis. Using magnetic resonance imaging, Franck et al. observed that the skin depression in IAPP lesions is solely because of dermal atrophy. [7] On the other hand, Abe et al., using B-mode ultrasound on one patient, attributed this morphology to both the dermal and the subcutaneous layers. [8]
The proper classification of atrophoderma has often been debated in the medical literature. Some authors regard atrophoderma as a distinct entity, characterized by early onset, long standing course, and absence of lilac margin; others suggest that IAPP is closely related to morphea, namely, a variant of morphea. The co-occurrence of IAPP and morphea has been reported. [9]
IAPP usually follows a benign course. Its lesions may be asymptomatically solitary or multiple patches, which progress very slowly and remain stable for 10-20 years. Most commonly, they are distributed symmetrically on the trunk, particularly on the back, and to a lesser extent on the extremities, with a few displaying a unilateral and zosteriform pattern. [1],[3],[10] However, Saleh et al. retrospectively reviewed all cases of IAPP who presented to the Department of Dermatology at the American University of Beirut between the years 1994 and 2006, and showed several new aspects of IAPP. Clinically, the lesions were most commonly hypopigmented and involved predominantly the extremities. [9]
The lesions in case 1 were involved in extensive regions, including the back, lumbar part, shoulders, and upper limbs. These lesions were asymptomatic, nonindurated, and lacked the characteristically inflamed border of morphea. They spread slowly over 10 years without showing a tendency to undergo regression. The lesions, varying in size from a few millimeters to several centimeters were oriented along the skin cleavage lines, but did not coalesce to form larger, irregularly shaped plaques. The absence of inflammation, sclerosis, or induration leads to a diagnosis of IAPP. To the best of our knowledge, our patient had the maximum number of lesions so far reported in the literature (>200). Considering the onset, course, the clinical and histological findings in this case, we support the concept that IAPP is a rare form of dermal atrophy and may represent a separate entity with an unclear cause.
Case 2 was also fascinating because the lesions displayed an extensive unilateral involvement occurring over a 7-year period. The lesion predominated over the right back and lumbar part, with a size of 27 Χ 23 cm. In contrast to previous reports, the involved area was the largest. Radiological examination displayed normal muscles and skeletons underlying the skin. More interestingly, this patient′s atrophic plaque contained two areas of indurated skin which were clinically compatible with morphea. The case implies that IAPP and morphea belong to a similar (nosologic) entity and IAPP is an abortive or superficial type of morphea.
In summary, our two cases seem to support the concept that two types of atrophoderma occur. Perceptibly, case 1 displayed a separate idiopathic entity of IAPP; however, case 2 presented coexistence of a giant lesion of IAPP and two morphea lesions arising within the atrophic hyperpigmented plaque. We believe that our observation adds a new clinical aspect to this peculiar condition.
No effective treatment for IAPP is known. However, psoralen and UVA (PUVA), potassium benzoic acid, and oral antibiotics may be helpful to some patients if B. burgdorferi antibodies are elevated. [6] Carter et al. suggested that hydroxychloroquine is a therapeutic option for chronic refractory APP. [11] In one case, the Q-switched alexandrite laser was effective in decreasing hyperpigmentation, but it did not help with atrophy. [12]
| 1. |
Canizares O, Sachs PM, Jaimovich L, Torres VM. Idiopathic atrophoderma of Pasini and Pierini. Arch Dermatol 1958;74:42-60.
[Google Scholar]
|
| 2. |
Kim SK, Rhee SH, Kim YC, Lee ES, Kang HY. Congenital atrophoderma of Pasini and Pierini. J Korean Med Sci 2006;21:169-71.
[Google Scholar]
|
| 3. |
Wakelin SH, James MP. Zosteriform atrophoderma of Pasini and Pierini. Clin Exp Dermatol 1995;20:244-6.
[Google Scholar]
|
| 4. |
Kernohan NM, Stankler L, Sewell HF. Atrophoderma of Pasini and Pierini. An immunopathologic case study. Am J Clin Pathol 1992;97:63-8.
[Google Scholar]
|
| 5. |
Tajima S, Sakuraoka K. A case of atrophoderma of Pasini and Pierini: Analysis of glycosaminoglycan of the lesional skin. J Dermatol 1995;22:767-9.
[Google Scholar]
|
| 6. |
Buechner SA, Rufli T. Atrophoderma of Pasini and Pierini: Clinical and histopathologic findings and antibodies to Borrelia burgdorferi in thirty-four patients. J Am Acad Dermatol 1994;30:441-6.
[Google Scholar]
|
| 7. |
Franck JM, MacFarlane D, Silvers DN, Katz BE, Newhouse J. Atrophoderma of Pasini and Pierini: Atrophy of dermis or subcutis? J Am Acad Dermatol 1995;32:122.
[Google Scholar]
|
| 8. |
Abe I, Ochiai T, Kawamura A, Muto R, Hirano Y, Ogawa M. Progressive idiopathic atrophoderma of Pasini and Pierini: The evaluation of cutaneous atrophy by 13-MHz B-mode ultrasound scanning method. Clin Exp Dermatol 2006;31:462-4.
[Google Scholar]
|
| 9. |
Saleh Z, Abbas O, Dahdah MJ, Kibbi AG, Zaynoun S, Ghosn S. Atrophoderma of Pasini and Pierini: A clinical and histopathological study. J Cutan Pathol 2008;35:1108-14.
[Google Scholar]
|
| 10. |
Miteva L, Kadurina M. Unilateral idiopathic atrophoderma of Pasini and Pierini. Int J Dermatol 2006;45:1391-3.
[Google Scholar]
|
| 11. |
Carter JD, Valeriano J, Vasey FB. Hydroxychloroquine as a treatment for atrophoderma of Pasini and Pierini. Int J Dermatol 2006;45:1255-6.
[Google Scholar]
|
| 12. |
Arpey CJ, Patel DS, Stone MS, Qiang-Shao J, Moore KC. Treatmemt of atrophoderma of Pasini and Pierini-associated hyperpigmentation with the Q-switched alexandrite laser: A clinical, histologic, and ultrastructural appraisal. Lasers Surg Med 2000;27:206-12.
[Google Scholar]
|
Fulltext Views
3,686
PDF downloads
1,657





