Translate this page into:
Cutaneous tuberculosis in children: The Indian perspective
Correspondence Address:
Archana Singal
B-14, Law Apartments, Karkardooma, Delhi - 110 092
India
| How to cite this article: Singal A, Sonthalia S. Cutaneous tuberculosis in children: The Indian perspective. Indian J Dermatol Venereol Leprol 2010;76:494-503 |
Abstract
Cutaneous tuberculosis continues to be a significant medical problem even with the advent of highly effective antituberculous drugs. It constitutes about 1.5% of all extra pulmonary tuberculosis. The prevalence in children varies from 18 to 54% in India. There is no gender predilection and the infection occurs with increased frequency in 10-14 year age group. Intrafamilial source of TB has been observed very frequently. A concomitant TB lymphadenitis is most common while involvement of other systemic organs like lung, bone and abdomen has also been observed. Protective efficacy of BCG is debatable and not yet fully defined. Of all the clinical types, scrofuloderma (SFD) is the most commonly encountered variant followed by lupus vulgaris (LV) and tuberculosis verrucosa cutis (TBVC). Lichen scrofulosorum (LS) is generally found to be associated with systemic TB focus in about 72% of cases. The impact of HIV on childhood cutaneous TB seems to be minimal. Similar to adults, the diagnosis of cutaneous tuberculosis relies mainly on histopathology, culture on LJ medium or radiometric BACTEC 460 TB culture system and PCR. In addition Mantoux positivity and a positive therapeutic trial with anti-tubercular drugs may be a good pointer to tubercular infection. A thorough clinical evaluation and exhaustive investigations to pin-point associated systemic focus is advocated as the latter has an impact on the duration of treatment. Cutaneous TB in children is treated as per the recommendations of therapy for extrapulmonary TB.Introduction
Despite the availability of effective chemotherapy and implementation of strategic health programs, tuberculosis (TB) still remains a formidable infection globally and an economic burden for resource-constrained countries. In 2007, India ranked first in terms of total number of TB cases (2.0 million) globally. [1] The persistent adverse social conditions, high rates of migration of infected people from areas of relatively high prevalence to low endemic areas, the co-existent human immunodeficiency virus (HIV) epidemic and the emergence of multidrug-resistant (MDR) Mycobacterium tuberculosis have contributed to the resurgence of TB as a major public health problem.
Childhood TB represents 5-15% of all cases of TB. Similar to adults, pulmonary TB is most common, with extrapulmonary disease occurring in 20% of the children. [2] Cutaneous TB accounts for about 1.5% of extrapulmonary cases. [3]
Epidemiology and Demographic Profile [Table - 1]
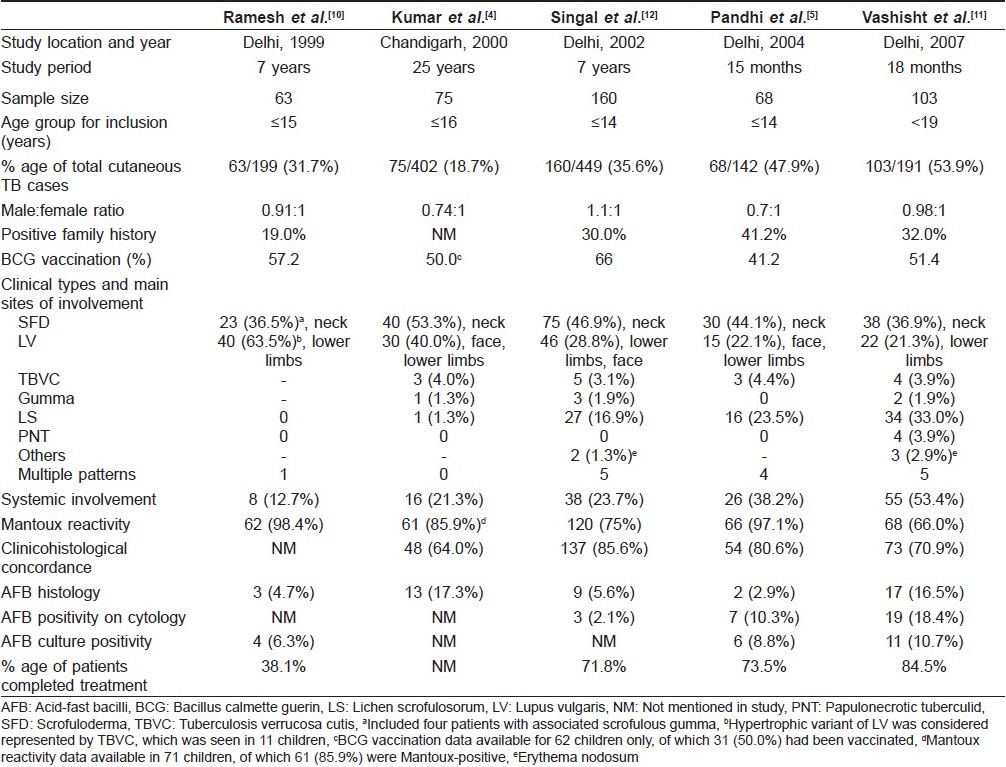
Cutaneous TB is caused by M. tuberculosis in a majority of cases and, rarely, by M. bovis. It accounts for 0.1-0.9% of the total dermatology out-patients in India. [3],[4],[5] The reported prevalence of cutaneous TB in children has varied in different studies depending on the geographical region. In older surveys from Hongkong, up to 50% of all cases of cutaneous TB were found in children, although now the overall incidence of the disease is very low in that country. [6],[7] In India, the prevalence of childhood skin TB has been reported to be 18.7% of all cases of skin TB in Chandigarh, 20.4% in Varanasi, 24.41% in Chennai and higher prevalence ranging from 31.7% to 53.9% in recent case series from Delhi. [4],[8],[9],[10],[11],[12] The difference in local prevalence of TB in certain parts of North India is suggested by a differential accumulation of cases over a period of time. In a case series from Delhi, Vashisht et al. reported 75 children diagnosed over 18 months and Pandhi et al. reported 68 children seen over just 15 months. [5],[11] However, Kumar et al. from Chandigarh and Ramesh et al. from Delhi reported similar number of cases (75 and 63 cases) detected over longer periods of 25 years and 7 years, respectively. [4],[10] Different upper limits for childhood case definition (ranging from <14 years to <19 years) in different studies might have also contributed to this statistical disparity.
Cutaneous TB affects the entire age spectrum in children. The incidence of disease in the 0-10 years age group has ranged widely from 12.8% in Delhi to 36.3% in Hongkong. [6],[13] However, a majority of the cases are seen in children 10-14 years of age, as reported by Kumar et al., Pandhi et al. and Singal et al0.[4],[5],[12] In most of the studies, both sexes were either equally affected or there was a marginal female preponderance, especially in cases of scrofuloderma (SFD). [4],[5],[10],[11],[12] The duration between the onset of lesions and detection in the hospital has widely ranged from 2 months to up to 10 years. Kumar et al. reported that the time interval to seek medical attention was within 1 year of the disease onset in most of the cases, although a delay of more than 3 years was seen in a few cases. [4] Such children are more likely to present with widespread or disseminated disease and remain as potential reservoirs of infection.
The source of skin TB in a child is often a household contact with present or past history of TB. History of TB (most commonly pulmonary) in a parent or close household relative in Delhi was found in 12 of 63 patients (19%) by Ramesh et al., 32% by Vashisht et al. and as high as 41.2% by Pandhi et al.[5],[10],[11] Most of the patients in the Indian series belong to families of low socioeconomic status with poor living conditions. Pandhi et al. reported that 69% of childhood cutaneous TB patients were residing in overcrowded dwellings. [5] Singal et al. have reported a case of widespread cutaneous TB with systemic involvement secondary to immunosuppression resulting from severe malnourishment in an Indian child. [14]
Cutaneous TB may remain localized to the skin alone, but frequently involves regional lymph nodes. Disease is considered to be disseminated if there is presence of generalized lymphadenopathy and/or involvement of other organ systems. [4] Compared to adults, children have a higher incidence of tuberculous lymphadenitis, seen in up to 29.2% of the cases. [11] Children are also more likely to have underlying systemic involvement compared to adults. Systemic involvement in children with skin TB in different case series has been reported to be present in 12.7-53.4% of the cases, with a higher incidence in cases with SFD. [4],[5],[10],[11] Of the extracutaneous sites involved, pulmonary infection is most common, seen in up to 20% of the cases, bone infection in up to 10% and abdominal TB in 5.8% of the children. [11] Concomitant involvement of the skin, lungs and bones has also been reported in a few cases.
BCG Vaccination and Cutaneous TB
At present, the role of BCG as a preventive measure against cutaneous TB is difficult to define. Protective efficacy of BCG vaccine in TB in various clinical trials has ranged from 0% to 80%. [15] In a meta-analysis, a high rate of 65-86% protection against tubercular meningitis and miliary disease was reported. [16] Zodpey et al. demonstrated BCG vaccination to be moderately effective in adults against skin TB, with an efficacy of 60.9%. [17] Kumar et al. reported that BCG-vaccinated children did not develop disseminated disease. [4] However, others found no significant differences between the vaccinated and the unvaccinated groups. [10],[12] Protective efficacy of BCG has been reported to decrease with time interval since vaccination.
Clinical Types
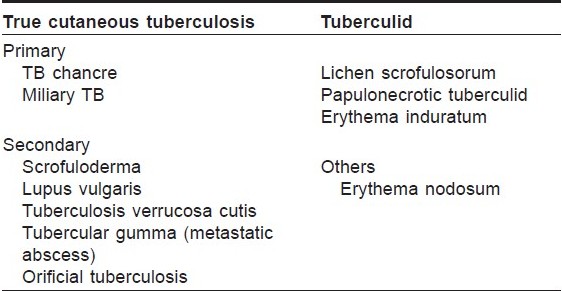
There are various classification systems of cutaneous TB in vogue. However, to simplify the understanding of the different types of cutaneous TB in children, they may be classified as true TB and tuberculids. [18] Depending on previous sensitisation, true TB may further be subdivided into primary or secondary types [Table - 2].
Scrofuloderma
SFD is the most common form of cutaneous TB seen in children. [4],[5],[11],[12] Vashisht et al. observed SFD in 36.9%, Singal et al. in 46.9% and Kumar et al. in 53.3% of the cases. [4],[11],[12] SFD arises due to direct extension of the infection from an underlying tuberculous focus into the skin. Lymph nodes are the most common foci, of which cervical node infection with SFD of the neck region is the most common site of involvement. [4],[5],[11],[12] This may possibly be due to the prevalent habit of drinking unboiled or unpasteurized milk in many parts of the country, with subsequent infection of the cervical lymph nodes. [4] Other lymph nodes may also be involved, including inguinal, axillary, submandibular, supraclavicular and epitrochlear. [18] Primary infection of the bone, including spinal TB, is another source of SFD in childhood. Rarely, intestinal Koch′s and hepatic TB may also lead to secondary cutaneous involvement. [5],[19] Multifocal SFD is also known to occur in children. [14] SFD usually starts as subcutaneous swellings or nodules, which rupture over a period of time to give rise to shallow ulcers with undermined edges [Figure - 1]. Formation of intermittently discharging sinus tracts occurs, which heal with characteristic puckered scarring. While biopsy from the periphery of the lesion may show granulomas suggestive of tubercular etiology, the histopathology is nonspecific in many cases. [4],[5] Caseation necrosis, ulceration and abscess formation are commonly encountered. Vashisht et al. observed that, compared with other forms of skin TB, SFD showed granulomatous involvement of the entire dermis, with giant cells as the predominant cell infiltrate. [11] SFD lesions are more likely to show presence of acid fast bacilli (AFB) from stained cytology smears or biopsy samples as well as positive cultures for mycobacteria compared with other types of cutaneous TB. [5],[11] The tuberculin test is usually positive.
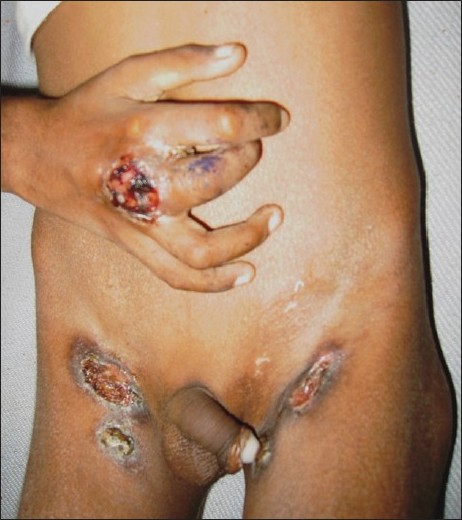 |
| Figure 1 :Scrofuloderma overlying the inguinal lymph nodes. Note: tubercular dactylitis of the right middle finger |
Lupus vulgaris
Lupus vulgaris (LV) is the most common clinical type of cutaneous TB in adults, and the second most common type seen in children. [4],[12] Ramesh et al. found it to be the most common form (63.5%) in their series. [10] It usually occurs in individuals previously sensitized to tubercular infection. The typical lesion is a well-demarcated skin-colored to erythematous plaque that shows evidence of healing and scarring in one area and activity in another [Figure - 2]. While Kumar et al. reported the face to be the most common site, overall, LV is mostly seen in the lower half of the body, involving legs, knees, thighs, buttocks and feet. [4],[5],[10],[11],[12] This has been attributed to the prevalent local habit of children playing without clothing and defecating in the open. [10] Involvement of the upper limbs and trunk is also known to occur, and multifocal cases have also been reported. [4],[10] While LV localized to the genitalia is rare in children, it often results in mutilation and severe disfigurement, especially of the vulva. [10] Contractures involving the knee, elbow and wrist and destruction of ear and nose [Figure - 3] are other rare disabling sequelae of LV in children. [5],[10] Many morphological variants have been described: classic plaque or keratotic type, hypertrophic, ulcerative, atrophic and planar. Of these, the keratotic type is the most common whereas ulcerative and atrophic forms are the least common in children. [5],[10],[11] Other unusual presentations, e.g. lesions in sporotrichoid pattern, occurrence at the site of BCG vaccination and in the vicinity of SFD have been rarely reported. [4],[11],[18] Although regional lymphadenopathy is commonly encountered in LV, systemic involvement is seen less often when compared with SFD. Histopathology of LV lesions reveals typical epithelioid granulomas in the upper dermis, with lymphocytes and Langhans giant cells in up to 80% of the cases, with the remainder showing nonspecific changes. [4],[5],[11] The hypertrophic form additionally shows the presence of epidermal hyperkeratosis, papillomatosis and acanthosis. Necrosis is usually not seen and fibrosis is evident in areas of healing and scarring. [11] AFB are scanty and difficult to detect by staining methods or mycobacterial culture. [18]
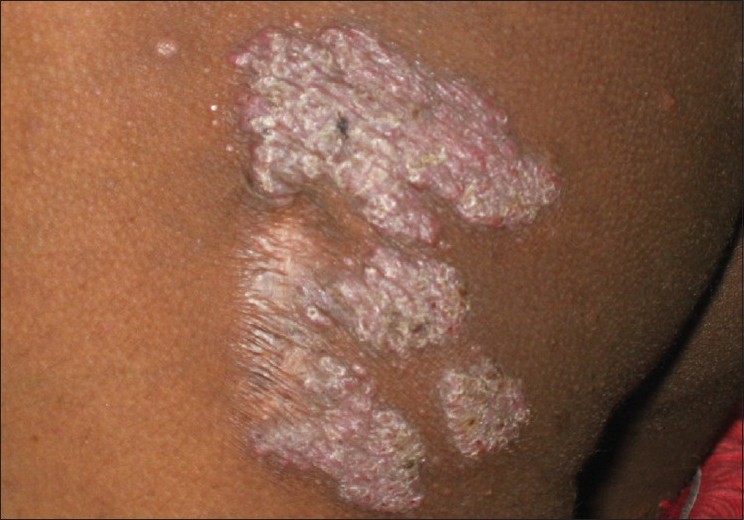 |
| Figure 2 :Erythematous, scaly plaque of lupus vulgaris with intervening atrophy and advancing margin on the left buttock |
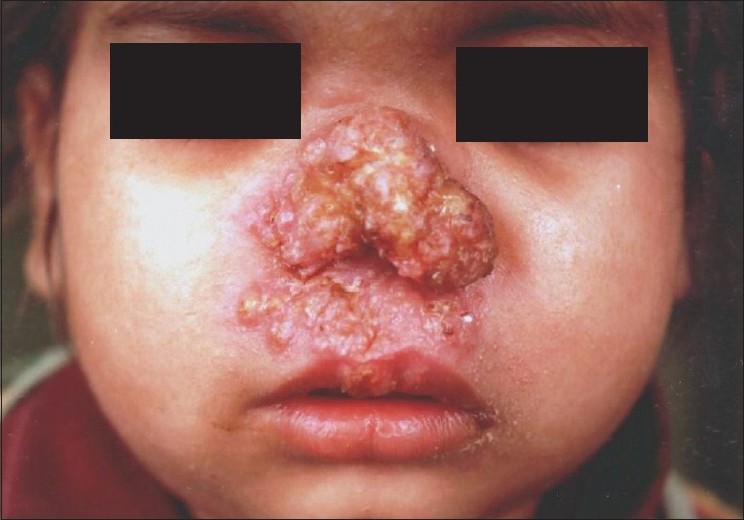 |
| Figure 3 :Mutilating lesion of lupus vulgaris over the nose in a young girl |
Tuberculosis verrucosa cutis
This form of the disease, also known as warty TB, occurs due to exogenous inoculation of tubercle bacilli in a previously sensitized individual with good immunity. Clinically, it presents as verrucous papules and plaques, with the surface showing fissures or clefts that may extrude pus and, often, perilesional erythema. It may be difficult to differentiate it from hypertrophic LV as well as verruca vulgaris or common warts. While tuberculosis verrucosa cutis (TBVC) was once seen in 65.5% of the childhood cases from Hongkong, it is an uncommon form of the disease in Indian children. [6] Although Arya et al. have reported TBVC in 15% of their patients (3/20), most of the other series from India have reported it to contribute to <4.5% of all cases of pediatric skin TB. [4],[5],[11],[20] Exposed body parts, in particular the lower limbs, are the most common site for warty TB in children, possibly due to inoculation by unnoticed trauma. In contrast to SFD and LV, lymphadenopathy is not seen. Histology shows hypertrophic changes like pseudoepitheliomatous hyperplasia, the presence of acute infiltrate in the upper dermis and characteristic tuberculoid granulomas in the mid dermis. [11] AFB are rarely demonstrated.
Tubercular gumma
Tubercular gumma or metastatic abscess results from disseminated infection by the hematogenous route. Clinically, it presents as dermal or subcutaneous nodules that soften to form fluctuant nontender abscesses and later break down to form sinuses or undermined ulcers. It is commonly seen in malnourished or immunosuppressed children. [17] This form has been infrequently reported in Indian children. [4],[10],[11],[12],[21] Presence of tubercles with widespread caseation necrosis and copious amounts of AFB is seen on histopathology.
Other types of "true cutaneous tuberculosis"
Primary inoculation TB or tubercular chancre occurs uncommonly due to exogenous infection in previously unsensitized children or as noduloulcerative lesions secondary to BCG inoculation in infants. [22],[23] Acute miliary TB caused by hematogenous dissemination of the organism with widespread internal involvement is also rarely seen in children, following exanthems or in disorders that predispose to poor immune status, like anhidrotic ectodermal dysplasia. [24],[25] Orificial TB manifesting as perianal tubercular ulcers has been reported in a 13-year-old boy from Tunisia. [26]
Tuberculids
A tuberculid is a cutaneous immunological reaction to the presence of occult TB in a patient with moderate to high immunity. The main features of tuberculid are the absence of organism in smears and negative mycobacterial culture, positive tuberculin test and rapid resolution of the lesions with antitubercular therapy. However, based on the detection of M. tuberculosis DNA by polymerase chain reaction (PCR), only lichen scrofulosorum (LS) may be considered to be true tuberculid, as PCR positivity has been observed in other tuberculids, i.e. papulonecrotic tuberculid and erythema induratum. [27]
Lichen scrofulosorum
LS is an infrequently reported but fairly common tuberculid occurring in patients with a systemic focus of TB and good immunity to the tubercle bacilli. It presents as grouped or clustered follicular papules, most commonly present on the trunk [Figure - 4]. Earlier studies have reported a low prevalence of LS in children. Kumar et al. and Ramesh et al. did not report seeing any patients in their childhood series. [4],[10] The low reporting of LS cases in earlier studies may be due to subtle and asymptomatic lesions getting missed by the patient as well as the physician and misdiagnosis as some other follicular disorder such as keratosis pilaris, lichen spinulosus, pityriasis rubra pilaris or lichen nitidus. [11] However, recent studies by Pandhi et al. and Vashisht et al. reported it to be the second most common pattern of cutaneous TB in children, occurring in 23.5% and 33% of the cases, respectively. [5],[11] A high index of suspicion might have been responsible for the higher reporting in these studies. In the largest published series of LS by Singal et al., 84% of the 39 patients were children <15 years of age. [28] The reason for this may be a decrease in delayed hypersensitivity response with increasing age.
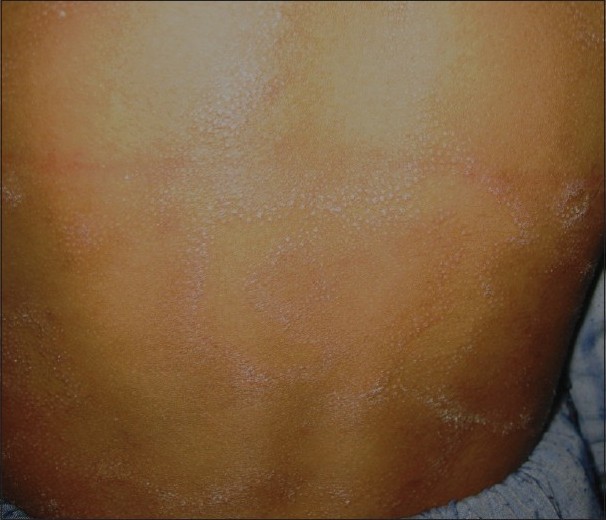 |
| Figure 4 :Erythematous, scaly and grouped lesions of lichen scrofulosorum over the back |
A systemic focus of TB is detected in a majority of LS cases, the most common being involvement of lymph nodes (cervical, mediastinal or hilar). [11] Singal et al. reported an associated focus of TB elsewhere in the body in 72% of the cases; 33% had tubercular lymphadenopathy while 28%, 8% and 15% had pulmonary TB, intracranial TB and other forms of cutaneous TB, respectively. [28] Fifteen percent had tubercular foci at multiple sites. Other foci detected in other studies include bones, joints and intestine or peritoneum. [11] Thus, a thorough systemic evaluation should be performed in all patients with confirmed or suspected LS. Apart from the trunk, LS may involve the axilla, groin, thighs, buttocks and arm. LS confined to the vulva, associated with cervical and inguinal tubercular lymphandenitis, has been reported in an 11-year-old girl. [29] Phlyctenular keratoconjunctivitis as a manifestation of immunological response to suspected TB antigen has also been reported with LS in a child with SFD/LV. [30]
Histology from lesions shows superficial perifollicular epithelioid granulomas without any caseation. [11],[28] AFB are not detected, mycobacterial cultures are negative from lesional biopsies and PCR is also negative. [28] Prompt and excellent response to antitubercular therapy is seen within 4-6 weeks, with complete clearance in 12 weeks, irrespective of the presence of tubercular focus elsewhere. [28]
Papulonecrotic tuberculids
These lesions are characterized by symmetrically distributed firm and dusky red necrotizing papules present predominantly over the extremities that heal with varioliform scarring. Jordaan et al. reported eight children with papulonecrotic tuberculids (PNT), of which seven had associated pulmonary TB and three had phlyctenular conjunctivitis. [31] It has also been reported in children with active tubercular lymphadenitis and disseminated LV. [32],[33] However, PNT has been infrequently reported in Indian children, with only four cases in the series by Vashisht et al0.[11] Histology shows wedge-shaped necrosis with nonspecific perivascular infiltrates or tubercular granulomas surrounding the necrosis. [11] Leukocytoclastic vasculitis, a histological feature characteristically present in PNT lesions in adults, has been unequivocally reported in children. While Vashisht et al. and Ramdial and colleagues found vasculitis as a prominent feature in their cases, Jordaan et al. did not observe it any of their children with PNT. [11],[31],[34] Although AFB are not demonstrable in papulonecrotic skin lesions, M. tuberculosis DNA is frequently detected by PCR. [35] Jordaan et al. have suggested that in cases where M. tuberculosis DNA can be confirmed by PCR, the term "papulonecrotic tuberculosis" should be preferred over "papulonecrotic tuberculid." [31]
Erythema induratum of Bazin (EIB)
This is another tuberculid characterized by subcutaneous nodules over the posterior legs, which break down into deep ulcers followed by crusting and finally heal with atrophic scarring. It is very uncommon in children, with only few cases reported till date and no childhood case from India. [36],[37],[38] Association with pulmonary TB is frequent and nodular episcleritis has been reported in a 6-year-old girl. [37],[38] Biopsy shows lobular panniculits with vasculitis and Mantoux test is strongly positive. M. tuberculosis DNA is detected by PCR in more than 50% of the cases. [39]
Erythema nodosum, characterized by painful subcutaneous nodules mainly on the shins, and septal panniculitis without vasculitis on histology, may also be associated with TB, especially in India. However, children are rarely affected. Vashisht et al. reported only three cases out of 103 and Singal et al. reported only two cases out of 160 children with cutaneous TB. [11],[12]
HIV and cutaneous TB
Pulmonary TB and certain extrapulmonary forms of TB are more common and extensive in HIV-infected patients. Cutaneous TB, including tubercular ulcers, PNT and disseminated miliary forms may be more severe in HIV-positives. [40] In various Indian series, none of the children with cutaneous TB tested positive for HIV by enzyme-linked immunosorbent assay. [5],[10],[11],[12] In a series of 231 patients from South India (adults as well as children), only two tested positive. [9] Thus, larger studies are required to assess the impact of HIV seropositivity on cutaneous TB.
Diagnosis
The diagnosis of cutaneous TB is based on the characteristic clinical morphology of the lesions as well as laboratory tests. Common clinical differential diagnoses of skin TB include cutaneous leishmaniasis, leprosy, atypical mycobacterial infections, fungal infections like chromomycosis and sporotrichosis and sarcoidosis. Many of these conditions also show granulomas on histology and thus definitive diagnosis relies on the direct demonstration of tubercle AFB on stained smears or biopsies, isolation by culture or detection by PCR and related molecular techniques.
Histopathology
The hallmark of cutaneous TB histology is the presence of characteristic tubercular granulomas with epithelioid cells, Langhans giant cells and lymphocytes. However, characteristics such as the distribution of granulomas in the dermis, nature of cellular infiltrate, presence of necrosis and certain specific epidermal changes aid in classifying and diagnosing the variants of skin TB, which have been discussed before (vide supra). But, the difficulty of histological interpretation in TB is well recognized as many other diseases can produce a tuberculoid granuloma. A clinicohistological concordance was observed in 64-85.6% of the cases of childhood cutaneous TB. [5],[11],[12] Classical tubercular histology is seen more often in lesions of LV compared with SFD. Histopathology is especially useful for diagnosis of tuberculids where bacilli cannot be isolated by culture or demonstrated by AFB staining.
Mantoux test
The Mantoux or tuberculin test is a good screening test to detect the presence or absence of tubercular infection. It is performed by injecting 0.1 ml (5 TU) of the purified protein derivative (PPD) on the volar surface of the forearm using a 27-gauge needle and measuring the induration after 48-72 h. Induration of 10 mm or more is considered significant, but indicates infection and not necessarily the disease. False-positive results may occur due to exposure to environmental mycobacteria as well as within 1 year of BCG vaccination. False-negative reactions may also be seen in patients with disseminated infection like miliary TB or in immunosuppressed states like severe malnutrition in children or HIV infection. Pandhi et al. reported 66 of 68 children (97.1%) with positive Mantoux test, with induration ranging from 10 to 32 mm. [5] Kumar et al. observed that of their children with cutaneous TB, 91.8% patients with localized disease had positive Mantoux test as against only 50% of the cases with disseminated disease. [4] Severe reactions resulting in vesiculation or ulceration have also been observed in children. [5],[11] Tuberculin test is more commonly positive in tuberculids, as reported in all cases of LS by Singal et al.[28]
Demonstration of AFB
Direct demonstration of the organism in tissue smear or biopsy specimens by staining for AFB is one of the most important tests for diagnosis of skin TB in children. Compared with culture by the traditional Lowenstein-Jenson (L-J) medium, direct AFB staining is inferior due to lesser sensitivity, but has the advantage of early availability of results. Vashisht et al. reported AFB positivity in 36.8% of the cases of SFD and 13.6% cases of LV. [11] In the same study, cytology smears showed 18.44% AFB positivity, with most of them being SFD. [11] Pandhi et al. additionally noticed that identification of AFB was higher (10.3%) in cytology smears from lymph nodes compared with detection on histological sections of skin biopsies (2.9%). [5] Authors suggested that culturing the organisms from cytology smears may be a good alternative in the future.
Culture
As for any other infectious disease, a positive culture for M. tuberculosis is considered to be the gold standard for the diagnosis of cutaneous TB. An additional use of culture is for testing drug susceptibility. Using the traditional L-J medium for culture, low rates of 8.8% and 10.7% positivity were reported by Pandhi et al. and Vashisht et al., respectively. [5],[11] The possible reason for overall low isolation of Mycobacteria could be that the bacilli are destroyed, nonviable or highly attenuated. [41] For some unexplained reasons, the culture positivity has been higher in adult cases. Owing to the tedious procedure of traditional culture systems, the focus is shifting to the use of culture methods like the radiometric BACTEC 460 TB culture system (Becton Dickinson Diagnostic Instrument Systems, Sparks, MD, USA) that is more rapid than the conventional culture procedures. [42] This medium uses Middlebrook 7H12 broth medium containing 14 C-labeled palmitic acid for the radiometric detection of mycobacterial growth. In a recent study, Aggarwal et al. reported the sensitivity of the BACTEC system to be much higher (62.8%) compared to the L-J medium (25.7%), with marked reduction in the mean detection time (17.3 vs. 39.4 days). [42] The combined isolation rate on both media was 74.3% (26/35), greater than that of either used separately. Similar results have been observed by Cutler et al., although their study involved <2% cutaneous TB cases. [43] The logical extrapolation of this principle would suggest that even in cutaneous TB, the "gold standard" for mycobacterial isolation would be a combination of both solid and liquid culture media.
QuantiFERON-TB Gold In-tube (QFT-IT)
This in vitro interferon-gamma-release assay is useful in the diagnosis of tubercular infection. Although it is considered to be superior to tuberculin skin testing owing to its higher specificity, the clinical experience with this test in the pediatric population is limited. [44] In fact, in a rural and predominantly BCG-vaccinated pediatric population in India, the tubercular skin test and QFT-IT assay were found to produce comparable results in the diagnosis of tubercular infection without any obvious advantage of the latter. [45] At present, the utility of this test is not well defined in cases of childhood cutaneous tuberculosis.
PCR
DNA amplification by PCR is another rapid, sensitive method that has been used to detect cutaneous TB in several studies, most commonly targeting the IS 6110 gene specific for the M. tuberculosis complex. However, PCR is a labor-intensive technique and is susceptible to several technical errors. It may provide false-positive results by carry-over contamination. False-negative results may be caused by degraded target DNA, the presence of PCR-inhibiting substances in clinical samples or insufficient extraction of target DNA. [42] Moreover, it cannot be used to test drug susceptibility. Finally, the high cost of this technique becomes prohibitive for its use, especially in a resource-poor country like India.
Antitubercular drug trial (Therapeutic trial)
The concept of therapeutic challenge as a valid diagnostic method in doubtful cases of cutaneous TB (where laboratory results are equivocal) has been corroborated in adults, and can be extended to the pediatric population as well. [46],[47] Four-drug antitubercular (ATT) therapy (consisting of isoniazid, rifampicin, pyrazinamide and ethambutol) is initiated and the clinical response is assessed in 4-6 weeks. Ramam et al. have suggested that 5 weeks appears to be an adequate duration of a therapeutic trial in patients suspected to have cutaneous TB. [48],[49] If there is no significant response by 5 weeks, it is unlikely that further treatment may be beneficial. [49] In such a case, either the diagnosis should be reviewed or possibility of MDR TB should be considered. However, patients with tuberculids and those with minimally active disease may take longer than 5 weeks to respond, and thus it may be worthwhile to prolong the therapeutic trial in such cases before considering alternative diagnoses. [49]
Systemic screening for tubercular focus
In all children, clinical assessment of lymph nodes, pulmonary and gastrointestinal system, nervous system, eyes and musculoskeletal system should be performed. Fine-needle aspiration cytology should be performed from the enlarged lymph nodes, and a part of the aspirate should be used for the culture of M. tuberculosis. Roentgenogram of chest and computed tomography (CT) scan (if required) should be performed and the sputum should be sent for AFB and mycobacterial culture. Because children have difficulty in producing sputum, it may be induced by saline nebulization. Children tend to swallow the sputum, and this can be retrieved as the gastric aspirate, which may be stained for AFB. Ultrasonographic assessment should be performed to screen for abdominal and pelvic TB. In select cases, imaging studies of the brain using CT scan or magnetic resonance imaging (MRI) may reveal intracranial tuberculoma. Assessment of cerebrospinal fluid is required only when tubercular meningitis is suspected. X-rays of the joints and bones suspected with tubercular osteomyelitis or arthritis and biochemical and microbiological evaluation of the joint aspirate may be performed. MRI spine is required to diagnose Pott′s spine in cases with paravertebral cold abscesses.
Treatment
Cutaneous TB is treated as per the recommendations of therapy for extrapulmonary TB. Apart from the investigations to establish the diagnosis of cutaneous TB, HIV testing should be carried out in all patients with confirmed or suspected TB because their HIV status makes a difference to their antitubercular treatment. [50] As per the latest recommendations (2009) of the World Health Organization (WHO), cutaneous TB in HIV-negative individuals (adults as well as children) should be treated by directly observed treatment short course (DOTS) chemotherapy consisting of four drugs, isoniazid (H), rifampicin (R), pyrazinamide (Z) and ethambutol (E) given for 2 months (intensive phase), followed by isoniazid and rifampicin given for the next 4 months (continuation phase). [50] Fixed-dose combinations (FDCs) should preferably be used. The drugs may be administered daily or three times weekly. The WHO recommends that daily dosing throughout the duration of therapy (2HRZE/4HR) is optimal for all newly diagnozed patients with TB. Alternatively, daily intensive phase followed by three-times weekly continuation phase [2HRZE/4(HR)3] or three-times weekly dosing throughout therapy [2(HRZE)3/4(HR)3] may also be used provided that every dose is directly observed. TB patients with known positive HIV status or living in an HIV-prevalent setting should receive daily doses of antitubercular drugs, at least during the intensive phase. [50] For the continuation phase, the optimal dosing frequency for such patients is also daily, although the three-times weekly dosing is an acceptable alternative.
In view of the growing evidence that the use of ethambutol is safe in young children, the WHO recommends that similar to adults, this drug should be used in all pediatric cases, irrespective of the age. [51] In a case of cutaneous TB where some systemic focus is detected, the corresponding disease categorization and recommendations should guide the choice of drugs and duration of therapy.
Adverse events caused by anti-TB drugs are much less common in children than in adults. [52] The most important adverse event is the development of hepatotoxicity, which can be caused by isoniazid, rifampicin or pyrazinamide. Because asymptomatic elevation of serum liver enzymes (less than five-times normal) is not an indication to stop treatment, they should not be monitored routinely. However, clinical signs such as liver tenderness, hepatomegaly or jaundice should prompt immediate stoppage of all potentially hepatotoxic drugs, assessment of serum liver enzyme levels and the patients should be screened for other causes of hepatitis. [52] No attempt should be made to reintroduce these drugs until liver functions have normalized. An expert should be involved in the further management of such cases. Isoniazid may cause symptomatic pyridoxine deficiency, especially in severely malnourished children and HIV-infected children on highly active antiretroviral therapy. Supplemental pyridoxine (5-10 mg/day) is recommended in malnourished children, HIV-infected children, breastfeeding infants and pregnant adolescents. [52]
| 1. |
Global tuberculosis control: Epidemiology, strategy, financing: WHO report 2009. Geneva, World Health Organization, 2009 (WHO/HTM/TB/2009.411).
[Google Scholar]
|
| 2. |
Kaur S, Thami GP, Kanwar AJ, Mohan H. Scrofuloderma with multiple organ involvement in a 5-year-old child. Pediatr Dermatol 2001;18:328-31.
[Google Scholar]
|
| 3. |
Kumar B, Muralidhar S. Cutaneous tuberculosis: a twenty-year prospective study. Int J Tuberc Lung Dis 1999;3:494-500.
[Google Scholar]
|
| 4. |
Kumar B, Rai R, Kaur I, Sahoo B, Muralidhar S, Radotra BD. Childhood cutaneous tuberculosis: A study over 25 years from northern India. Int J Dermatol 2001;40:26-32.
[Google Scholar]
|
| 5. |
Pandhi D, Reddy BS, Chowdhary S, Khurana N. Cutaneous tuberculosis in Indian children: The importance of screening for involvement of internal organs. J Eur Acad Dermatol Venereol 2004;18:546-51.
[Google Scholar]
|
| 6. |
Wong KO, Lee KP, Chiu SF. Tuberculosis of the skin in Hong Kong. A review of 160 cases. Br J Dermatol 1968;80:424-9.
[Google Scholar]
|
| 7. |
Chong LY, Lo KK. Cutaneous tuberculosis in Hong Kong: A 10-year retrospective study. Int J Dermatol 1995;34:26-9.
[Google Scholar]
|
| 8. |
Singh G. Lupus vulgaris in India. Indian J Dermatol Venereol Leprol 1974;40:257-60.
[Google Scholar]
|
| 9. |
Umapathy KC, Begum R, Ravichandran G, Rahman F, Paramasivan CN, Ramanathan VD. Comprehensive findings on clinical, bacteriological, histopathological and therapeutic aspects of cutaneous tuberculosis. Trop Med Int Health 2006;11:1521-8.
[Google Scholar]
|
| 10. |
Ramesh V, Misra RS, Beena KR, Mukherjee A. A study of cutaneous tuberculosis in children. Pediatr Dermatol 1999;16:264-9.
[Google Scholar]
|
| 11. |
Vashisht P, Sahoo B, Khurana N, Reddy BS. Cutaneous tuberculosis in children and adolescents: A clinicohistological study. J Eur Acad Dermatol Venereol 2007;21:40-7.
[Google Scholar]
|
| 12. |
Singal A, Mohanty S, Gandhi V, Bhattacharya S. Cutaneous tuberculosis in paediatric age group. In Proceedings; 7 th Congress of European Society for Paediatric Dermatology; 2002. p. 33-4.
[Google Scholar]
|
| 13. |
Sehgal VN, Jain MK, Srivastava G. Changing pattern of cutaneous tuberculosis. A prospective study. Int J Dermatol 1989;28:231-6.
[Google Scholar]
|
| 14. |
Singal A, Pandhi D, Agrawal SK. Multifocal scrofuloderma with disseminated tuberculosis in a severely malnourished child. Pediatr Dermatol 2005;22:440-3.
[Google Scholar]
|
| 15. |
Fine PE, Rodrigues LC. Modern vaccines: mycobacterial disease. Lancet 1990;1:1016-20.
[Google Scholar]
|
| 16. |
Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: A meta-analysis. Int J Epidemiol 1993;22:1154-8.
[Google Scholar]
|
| 17. |
Zodpey SP, Shrikhande SN, Maldhure BR, Kulkarni SW. Effectiveness of Bacillus Calmette Guerin (BCG) vaccination in the prevention of tuberculosis of skin: A case control study. Indian J Dermatol 1998;43:4-6.
[Google Scholar]
|
| 18. |
Sethuraman G, Ramesh V, Ramam M, Sharma VK. Skin tuberculosis in children: learning from India. Dermatol Clin 2008;26:285-94.
[Google Scholar]
|
| 19. |
Gautam A, Singh JP. Isolated hepatic tuberculosis with scrofuloderma. Postgrad Med J 1987;63:401-2.
[Google Scholar]
|
| 20. |
Arya L, Koranne RV, Manorama D. Cutaneous tuberculosis in children a clinico-microbiological study. Indian J Dermatol Venereol Leprol 1999;65:137-9.
[Google Scholar]
|
| 21. |
Premalatha S, Rao NR, Somasundaram V, Abdul Razack EM, Muthuswami TC. Tuberculous gumma in sporotrichoid pattern. Int J Dermatol 1987;26:600-1.
[Google Scholar]
|
| 22. |
Pillai SMS, Sarojini PA. Primary inoculation tuberculosis. Indian J Dermatol Venereol Leprol 1988;54:97-8.
[Google Scholar]
|
| 23. |
Paul MA, Williford PM. Cutaneous tuberculosis in a child: case report and review. Pediatr Dermatol 1996;13:386-8.
[Google Scholar]
|
| 24. |
Kakakhel KU, Fritsch P. Cutaneous tuberculosis. Int J Dermatol 1989;28:355-62.
[Google Scholar]
|
| 25. |
Frix CD 3rd, Bronson DM. Acute miliary tuberculosis in a child with anhidrotic ectodermal dysplasia. Pediatr Dermatol 1986;3:464-7.
[Google Scholar]
|
| 26. |
Mlika RB, Tounsi J, Fenniche S, Hajlaoui K, Marrak H, Mokhtar I. Childhood cutaneous tuberculosis: A 20-year retrospective study in Tunis. Dermatol Online J 2006;12:11.
[Google Scholar]
|
| 27. |
Lucas S. Bacterial diseases. In: Elder DE, Elenitsas R, Johnson BL, Murphy GF, editors. Lever's Histopathology of the Skin. 9 th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. p. 551-90.
th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. p. 551-90.'>[Google Scholar]
|
| 28. |
Singal A, Bhattacharya SN. Lichen scrofulosorum: A prospective study of 39 patients. Int J Dermatol 2005;44:489-93.
[Google Scholar]
|
| 29. |
Pandhi D, Mehta S, Singal A. Genital tuberculid in a female child: A new entity (childhood vulval tuberculid). Pediatr Dermatol 2007;24:573-5.
[Google Scholar]
|
| 30. |
Singal A, Aggarwal P, Pandhi D, Rohatgi J. Cutaneous tuberculosis and phlyctenular keratoconjunctivitis: A forgotten association. Indian J Dermatol Venereol Leprol 2006;72:290-2.
[Google Scholar]
|
| 31. |
Jordaan HF, Schneider JW, Schaaf HS, Victor TS, Geiger DH, Van Helden PD, et al. Papulonecrotic tuberculid in children. A report of eight patients. Am J Dermatopathol 1996;18:172-85.
[Google Scholar]
|
| 32. |
Chavalittamrong B, Chantrarakul N, Talalak P, Bedavanija A. Papulonecrotic tuberculid in childhood. Southeast Asian J Trop Med Public Health 1980;11:395-8.
[Google Scholar]
|
| 33. |
Senol M, Ozcan A, Aydin A, Karincaoglu Y, Sasmaz S, Sener S. Disseminated lupus vulgaris and papulonecrotic tuberculid: Case report. Pediatr Dermatol 2000;17:133-5.
[Google Scholar]
|
| 34. |
Ramdial PK, Mosam A, Mallett R, Aboobaker J. Papulonecrotic tuberculid in a 2-year-old girl: With emphasis on extent of disease and presence of leucocytoclastic vasculitis. Pediatr Dermatol 1998;15:450-5.
[Google Scholar]
|
| 35. |
Victor T, Jordaan HF, Van Niekerk DJ, Louw M, Jordaan A, Van Helden PD. Papulonecrotic tuberculid. Identification of Mycobacterium tuberculosis DNA by polymerase chain reaction. Am J Dermatopathol 1992;14:491-5.
[Google Scholar]
|
| 36. |
Lighter J, Tse DB, Li Y, Borkowsky W. Erythema induratum of Bazin in a child: Evidence for a cell-mediated hyper-response to Mycobacterium tuberculosis. Pediatr Infect Dis J 2009;28:326-8.
[Google Scholar]
|
| 37. |
Leahy TR, Downey P, Ramsay B, Philip RK. Erythema induratum of Bazin and episcleritis in a 6 year old girl. Arch Dis Child 2005;90:1132.
[Google Scholar]
|
| 38. |
Jordaan HF, Schneider JW, Abdulla EA. Nodular tuberculid: a report of four patients. Pediatr Dermatol 2000;17:183-8.
[Google Scholar]
|
| 39. |
Tan SH, Tan HH, Sun YJ, Goh CL. Clinical utility of polymerase chain reaction in the detection of Mycobacterium tuberculosis in different types of cutaneous tuberculosis and tuberculids. Ann Acad Med Singapore 2001;30:3-10.
[Google Scholar]
|
| 40. |
Hay RJ. Cutaneous infection with Mycobacterium tuberculosis: how has this altered with the changing epidemiology of tuberculosis? Curr Opin Infect Dis 2005;18:93-5.
[Google Scholar]
|
| 41. |
Ramesh V, Misra RS, Jain RK. Secondary tuberculosis of the skin. Clinical features and problems in laboratory diagnosis. Int J Dermatol 1987;26:578-81.
[Google Scholar]
|
| 42. |
Aggarwal P, Singal A, Bhattacharya SN, Mishra K. Comparison of the radiometric BACTEC 460 TB culture system and Lφwenstein-Jensen medium for the isolation of mycobacteria in cutaneous tuberculosis and their drug susceptibility pattern. Int J Dermatol 2008;47:681-7.
[Google Scholar]
|
| 43. |
Cutler RR, Baithun SI, Doran HM, Wilson P. Association between the histological diagnosis of tuberculosis and microbiological findings. Tuber Lung Dis 1994;75:75-9.
[Google Scholar]
|
| 44. |
Grare M, Derelle J, Dailloux M, Laurain C. Difficulties of TB diagnosis in children: QuantiFERON TB Gold In-Tube as useful tool. Arch Pediatr 2010;17:77-85.
[Google Scholar]
|
| 45. |
Dogra S, Narang P, Mendiratta DK, Chaturvedi P, Reingold AL, Colford JM Jr, et al. Comparison of a whole blood interferon-gamma assay with tuberculin skin testing for the detection of tuberculosis infection in hospitalized children in rural India. J Infect 2007;54:267-76.
[Google Scholar]
|
| 46. |
Sehgal VN, Sardana K, Bajaj P, Bhattacharya SN. Tuberculosis verrucosa cutis: Antitubercular therapy, a well-conceived diagnostic criterion. Int J Dermatol 2005;44:230-2.
[Google Scholar]
|
| 47. |
Sehgal VN, Sardana K, Sehgal R, Sharma S. The use of anti-tubercular therapy (ATT) as a diagnostic tool in pediatric cutaneous tuberculosis. Int J Dermatol 2005;44:961-3.
[Google Scholar]
|
| 48. |
Ramam M, Mittal R, Ramesh V. How soon does cutaneous tuberculosis respond to treatment? Implications for a therapeutic test of diagnosis. Int J Dermatol 2005;44:121-4.
[Google Scholar]
|
| 49. |
Ramam M, Tejasvi T, Manchanda Y, Sharma S, Mittal R. What is the appropriate duration of a therapeutic trial in cutaneous tuberculosis? Further observations. Indian J Dermatol Venereol Leprol 2007;73:243-6.
[Google Scholar]
|
| 50. |
Treatment of tuberculosis: guidelines. 4 th ed. Geneva: World Health Organization, 2009 (WHO/HTM/TB/2009.420).
[Google Scholar]
|
| 51. |
Donald PR, Maher D, Maritz JS, Qazi S. Ethambutol dosage for the treatment of children: literature review and recommendations. Int J Tuberc Lung Dis 2006;10:1318-30.
[Google Scholar]
|
| 52. |
Stop TB Partnership Childhood TB Subgroup, World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Chapter 2: Anti-tuberculosis treatment in children. Int J Tuberc Lung Dis 2006;10:1205-11.
[Google Scholar]
|
Fulltext Views
10,890
PDF downloads
2,756





