Translate this page into:
Autosomal dominant epidermodysplasia verruciformis: A clinicotherapeutic experience in two cases
Correspondence Address:
Vikram K Mahajan
Department of Dermatology, Venereology & Leprosy, Dr. R.P. Govt. Medical College, Kangra (Tanda) -176 001, Himachal Pradesh
India
| How to cite this article: Vohra S, Sharma NL, Shanker V, Mahajan VK, Jindal N. Autosomal dominant epidermodysplasia verruciformis: A clinicotherapeutic experience in two cases. Indian J Dermatol Venereol Leprol 2010;76:557-561 |
Abstract
Epidermodysplasia verruciformis (EV) is a rare genodermatosis characterized by a unique susceptibility to cutaneous infection by a group of phylogenetically related human papilloma viruses (HPVs). These patients show a defect in cell-mediated immunity specific toward the causative HPVs that lead to lifelong disease. The defect is usually inherited as autosomal recessive trait and presents clinically with plane warts, pityriasis versicolor-like lesions and reddish verrucous plaques. Dysplastic and malignant changes in the form of actinic keratoses, Bowen's disease and squamous cell carcinoma (SCC) are common but metastasis occurs rarely. A totally effective treatment against EV is as yet highly desirable. Two siblings having autosomal dominant EV presented with multiple actinic keratoses in addition to classic lesions. One of them had also developed well-differentiated SCC over forehead with metastases to regional lymph nodes. They were treated with combination of excision of small malignant/premalignant lesions, topical 5-flurouracil and sun protection. Additionally, elective excision/grafting of large SCC was performed after chemotherapy/radiotherapy in patient with metastatic SCC. Oral acitretin (25 mg/day) was of benefit in the other patient. Overall clinicotherapeutic experience in both the patients is discussed here.Introduction
Epidermodysplasia verruciformis (EV) is a rare genodermatosis characterized by wide spread and persistent infection with human papilloma viruses (HPVs), presenting clinically with characteristic combination of pityriasis versicolor-like lesions, reddish verruca-like and seborrheic keratosis-like plaques with a potential for malignant transformation. This malignant transformation largely depends upon the oncogenic potential of the infecting virus. To date >100 HPV types have been identified but mostly HPV 3, 5, 8, 9, 10, 12, 14, 17, 19-25, 28 and 29 are related to EV [1],[2],[3] and more than one HPV type may be present simultaneously in the same patient. HPV 5 and 8 are the main types incriminated for the malignant transformation in about 25% of EV patients. [2],[3] These patients show a defect of cell-mediated immunity that is specific toward the causative HPVs. This inability to recognize and reject HPVs leads to lifelong lesions/disease. Interestingly, these EV associated HPVs have been also involved in both benign and malignant proliferation of keratinocytes, as in the early stages of carcinogenesis. [4] The convincing oncogenic potential of HPV 5 and HPV 8 in cutaneous malignancies of EV patients [5] has evoked a considerable interest among clinicians and basic researchers for this rare genetic disorder as a model of cutaneous carcinogenesis.
EV occurs worldwide but does not display any predisposition in terms of gender or geographic distribution. Its frequency is 11% in Europe and USA and 40% among Japanese, [6] but reported infrequently from India. A PubMed and IndMed search on 26 Oct 2009 revealed only 12 reports appearing between 1971 and 2009. [6],[10],[11],[12],[13],[14],[15],[16],[17] In this paper we describe two new EV cases with an idea of their documentation and to share our clinicotherapeutic experience.
Case Reports
Case 1
A 35-year-old female born of non-consanguineous marriage presented with asymptomatic mildly erythematous and hypopigmented, pityriasis versicolor-like lesions [Figure - 1]A over the abdomen and extremities since the age of 8 years, and multiple brownish-black hyperkeratotic warty papuloplaque lesions of actinic keratosis over face, V-area of neck [Figure - 1]B and C, dorsal hands and feet. These lesions had appeared over a period of time and were progressively increasing in number and size. A large nodule over the right side of forehead had developed a non-healing ulcer for the last 2 years. It was nontender, hard in consistency, fixed, had hemorrhagic crusting and rolled out margins extending on to the eyebrow [Figure - 1]D. The preauricular, submandibular and anterior cervical lymph nodes were enlarged, hard and fixed to the underlying structures. The hair, nails, mucous membranes and other systemic examination showed no abnormality. Her two children, 5-month and 6-year-old, were normal. Except for her 21-year-old brother (case 2), other five siblings were reportedly healthy. The histology of the forehead ulcerated nodule was consistent with well-differentiated squamous cell carcinoma (SCC). Histopathology of a hypopigmented lesion showed features of plane wart with dysplasia [Figure - 2]. Fine needle aspiration cytology from right preauricular lymph node revealed irregular tightly cohesive cluster as well as isolated population of tumor epithelial cells showing pleomorphism, high nuclear to cytoplasmic ratio, hyperchromatic nuclei and abundant deeply basophilic cytoplasm. Computed tomography (CT) scan of head and neck showed multiple enlarged lymph nodes bilaterally in station-2 (between angle of mandible and lower border of hyoid bone) and station-3 (between lower border of hyoid bone and lower border of cricoid cartilage). There was no other clinico-investigative evidence of underlying bone involvement or distant metastasis. Other laboratory investigations including complete blood counts, serum biochemistry, HIV serology and urinalysis were essentially normal.
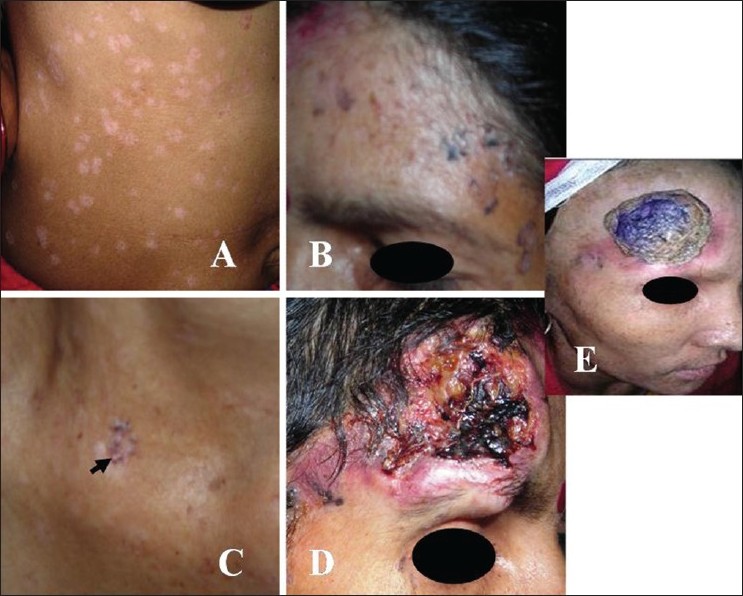 |
| Figure 1 :Case 1: (A) Pityriasis versicolor-like lesions over abdomen; (B and C) actinic keratoses on forehead and V-area neck (arrow), (D) nodulo-ulcerative SCC on forehead extending up to eye, (E) regression of tumor and lymph nodes, 12 weeks after chemo-radiotherapy. Note: Anagen effluvium |
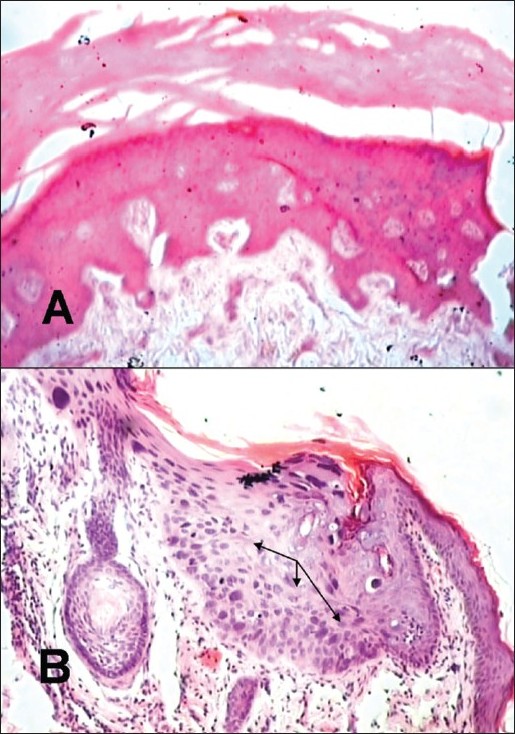 |
| Figure 2 :Case 1: (A) Histopathology of a hypopigmented lesion showing marked hyperkeratosis and focal basket-weave appearance and papillomatosis, (H and E, ×10), (B) showing mild acanthosis, hypergranulosis with few cells having vacuolation, marked condensation of chromatin and prominent eosiniophilic nucleoli, and occasional dyskeratotic cells in the dermis suggestive of wart with dysplasia (arrows indicate area of dysplasia), (H and E, ×40). Note: Similar histologic changes were also observed in a similar lesion in case 2 |
A diagnosis of EV and SCC with metastasis to regional lymph nodes was made. Topical 5-flurouracil (5FU) 5% was prescribed for twice daily application over small (<5 mm) lesions of actinic keratoses, while the large (>5 mm) lesions were excised surgically with primary closure. Liberal use of sun barriers/topical sunscreens was advised. The excision of large SCC was postponed because of its close proximity to eye and she was put under oncologist′s care. She received chemotherapy comprising parenteral cisplatin (40 mg/m 2 ) and 5-FU (750 mg) on two consecutive days, repeated at an interval of 21 days. Except for the development of anagen effluvium, the chemotherapy was well tolerated. After three cycles of chemotherapy, she was treated with a fractionated radiotherapy (60 gray 30 fractions) for 6 weeks. Following chemo-radiotherapy induced regression of tumor and lymph nodes[Figure - 1]E, wide surgical excision with temporoparietal rotation flap grafting for primary lesion was performed.
Case 2
The 21-year-old brother of case 1 presented with similar cutaneous lesions since the age of 6 years. The lesions were asymptomatic, mildly erythematous pityriasis versicolor-like over chest and upper extremities, multiple verrucae on dorsal hands, and actinic keratoses on face and V-area of neck with crusting and bleeding in some of them [Figure - 3] A-C. All these lesions were progressively increasing in number and size. The histology of a lesion excised from front of neck was that of actinic keratosis. The biopsy from the lesion on dorsal hand was consistent with plane wart having focal cellular atypia. Other systemic examination and laboratory investigations (as in case 1) revealed no abnormality.
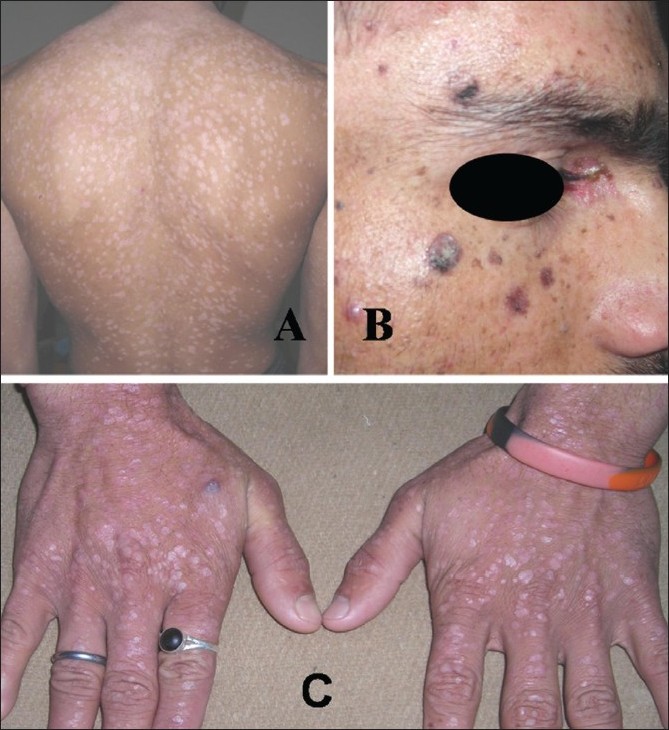 |
| Figure 3 :Case 2: (A) Pityriasis versicolor-like lesions on trunk, (B) actinic keratoses on face, (C) multiple verrucae on dorsal hands |
The large lesions of actinic keratoses were excised surgically. Topical 5-FU (5%) was prescribed for the smaller lesions. He was put on oral acitretin 25 mg/day and advised to use sun barriers/sunscreens liberally. During 1 year of follow up, partial resolution of facial lesions and flattening of warty lesions over hand without recurrence was observed [Figure - 4]. Topical 5-FU was also well tolerated and resulted in clearance of actinic keratoses lesions. He is under observation.
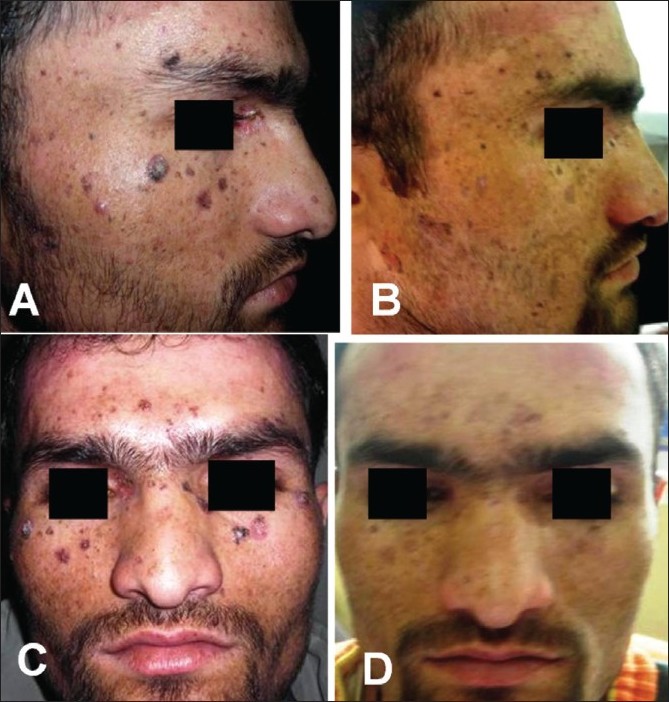 |
| Figure 4 :Case 2: (A and C) Before treatment and (B and D) 12 weeks after treatment, showing partial resolution of facial actinic keratoses lesions |
Discussion
The diagnosis of EV as well as cutaneous malignancy is mainly clinicopathologic while DNA amplification studies may be needed for identification of HPVs′ types. The hallmark lesions of EV are preferentially located on sun-exposed sites of face, neck, trunk and extremities. The malignant transformation of EV lesions into SCC occurs in 30-70% cases; this may require over 20 years for such conversion or occurs when the lesions have been present for 10 years or so, or usually in third and fourth decades of life. [18],[19],[20] It has been suggested that actinic damage is perhaps the pathogenic mechanism involved in malignant transformation of lesions over extremities and forehead. [18],[21] However, bowenoid lesions over vulva and/or periungual skin, body parts not directly exposed to sun, have been documented and are attributed due to physical trauma. [15],[22] Both our patients presented with classic lesions of the disorder, some of them had already become malignant, and histopathology correlated well with the clinical diagnosis of different lesions. HPV typing could not be carried out for want of facility/expense involved. Histologically, cellular atypia in plane wart lesions raises the possibility of these being premalignant themselves as has also been suggested by Kuruvilla et al.[14] The dysplastic and malignant changes in the lesions usually occur in the form of actinic keratosis, Bowen′s disease or SCC having a low metastatic potential. [18] However, all types of lesions seen in EV patients should be subjected to histopathology for early detection of any malignant changes, as clinically benign appearing lesions can demonstrate atypia. Early identification and appropriate treatment of actinic keratoses is also important as these are increasingly being recognized as an early clinical manifestation of biological continuum that may ultimately lead to invasive SCC. Our case 1 had multiple actinic keratoses and had developed SCC over forehead with metastases to regional lymph nodes, per se a rare occurrence and to the best of our knowledge not documented in Indian patients reported previously. [10],[11]
Stringent sun protection and lifelong observation for early diagnosis of malignant/premalignant lesions, which then can be treated early with surgical excision/grafting or ablated locally, is imperative for improved prognosis/survival of EV patients. The Mohs′ micrographic surgery will preserve healthy tissue which is important in these patients having multiple skin cancers and risk of recurrences. The role of etretinate in EV is not clear as signs of viral infection persist pathologically despite substantial clinical improvement in patients treated with etretinate 1 mg/kg body weight/day. [23] Acitretin 0.5-1 mg/day too has been effective and is perhaps the drug of choice currently. [24] Topical 5-FU and acitretin 25 mg/day were useful in our case 2. However, these non-surgical modalities, which are only palliative and improve quality of life, need large controlled studies for recommending their lifelong use. Metastasis to regional lymphnodes may have been observed rarely; nevertheless, it does occur and needs a multidisciplinary management for improved prognosis. Whether radiotherapy causes acceleration of malignant transformation, as has been suggested, [18] can however be known only after long-term follow up of these patients. However, chemotherapy with or without radiotherapy, as in our case 1, may be useful to achieve regression of the tumor/lymph nodes before surgery.
Association of EV with combined variable immunodeficiency syndrome, IgM deficiency and HIV infection has been well documented. [16],[25],[26] However, the underlying defect in EV is still not well understood. This susceptibility of human keratinocytes to the HPV in EV is usually inherited through an autosomal recessive gene and pathogenic mutations have been detected in EVER1/TMC6 and EVER2/TMC8 genes mapped to a locus on chromosome 17q 25 that encodes integral membrane proteins of unknown significance in the endoplasmic reticulum. [27] A high frequency of parental consanguinity is usual in most reported cases and more than 30% of the siblings acquire the disease at some or the other stage. [6] Nevertheless, cases with autosomal dominant or X-linked inheritance too have been reported. [28] There was no evidence of immunosuppression in our patients and, in the absence of parental consanguinity, the pattern of inheritance appears autosomal dominant [Figure - 5]. The absence of the disorder in either of the parents, unusual for disorders with autosomal dominant inheritance, can be explained on the basis of "lack of penetrance" wherein a person inheriting the defective gene may not develop the disorder.
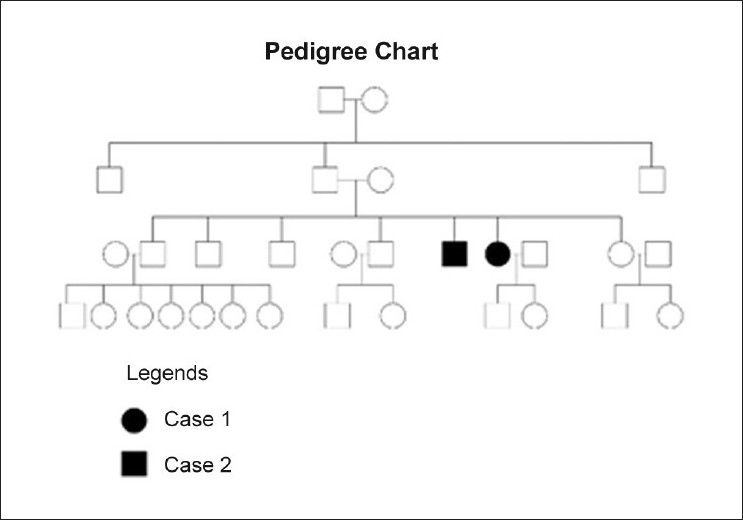 |
| Figure 5 :Pedigree chart of EV patients |
Acknowledgment
The help extended by our colleagues from Oncology (Head and Neck) Department in managing these patients is gratefully acknowledged.
| 1. |
Garcνa-Rνo I, Garcia-F-Villalta MJ, Daudιn E,Fraga J, Garcνa-Dνez A. Epidermodysplasia verruciformis-like lesions in a patient with systemic lupus erythematosus. Acta Derm Venereol 2003;83:229-30.
[Google Scholar]
|
| 2. |
Ostrow RS, Manias D, Mitchell AJ, Stawowy L, Faras AJ. Epidermodysplasia verruciformis. A case with primary lymphatic dysplasia, depressed cell-mediated immunity, and Bowen's disease containing human papilloma 16 DNA. Arch Dermatol 1987;123:1511-6.
[Google Scholar]
|
| 3. |
Pfister H. Human papilloma viruses and impaired immunity vs epidermodysplasia verruciformis. Arch Dermatol 1987;123:1469-70.
[Google Scholar]
|
| 4. |
Favre M, Majewski S, Noszczyk B, Maienfisch F, Pura A, Orth G, et al. Antibodies to human papilloma virus type 5 are generated in epidermal repair processes. J Invest Dermatol 2000;114:403-7.
[Google Scholar]
|
| 5. |
Majewski S, Jabloska S. Epidermodysplasia verruciformis as a model of human papilloma-induced genetic cancer of the skin. Arch Dermatol 1995;131:1312-8.
[Google Scholar]
|
| 6. |
Sehgal VN, Luthra A, Bajaj P. Epidermodysplasia verruciformis: 14 members of a pedigree with intriguing squamous cell carcinoma. Int J Dermatol 2002;41:500-3.
[Google Scholar]
|
| 7. |
Shah BH, Jalan VD. Review of literature of epidermodysplasia verruciformis with 2 case reports. Indian J Dermatol Venereol Leprol 1971;37:16-8.
[Google Scholar]
|
| 8. |
Singh OP, Nayyar KC. Epidermodysplasia verruciformis. Indian J Dermatol Venereol Leprol 1973;39:26-8.
[Google Scholar]
|
| 9. |
Vasishtha LK. Condylomata accuminata like lesions in epidermodysplasia verruciformis. Indian J Dermatol Venereol Leprol 1981;47:229-31.
[Google Scholar]
|
| 10. |
Sharma VK, Kaur S, Kumar B, Sehgal S. Epidermodysplasia verruciformis with squamous cell carcinoma in a family. Indian J Dermatol Venereol Leprol 1985;51:80-6.
[Google Scholar]
|
| 11. |
Bandyopadhyay D. Epidermodysplasia verucciformis with multiple squamous cell carcinomata. Indian J Dermatol 1995;40:102-3.
[Google Scholar]
|
| 12. |
Grover S, Sayal SK, Singh G, Dash K. Epidermodysplasia verruciformis. Indian J Dermatol Venereol Leprol 1996;62:61.
[Google Scholar]
|
| 13. |
Ramesh A, Krishanan SG, Jayaraman M, Janaki VR, Boopalraj JM. Epidermodysplasia verruciformis. Indian J Dermatol Venereol Leprol 1997;63:238-40.
[Google Scholar]
|
| 14. |
Kuruvilla M, Raghuveer CV, Rao GS, Sridhar KS, Kumar P. Epidermodysplasia verruciformis with bowenoid changes. Indian J Dermatol Venereol Leprol 1998;64:195-6.
[Google Scholar]
|
| 15. |
Sentamilselvi G, Lakshmi S, Sampath V, Janki C, Janaki VR. Epidermodysplasia verruciformis with vulval bowen's disease. Indian J Dermatol 2001;46:161-2.
[Google Scholar]
|
| 16. |
Karthikeyan K, Thappa DM, Ghotekar LH. Epidermodysplasia verruciformis with HIV infection. Indian J Dermatol 2004;49:160-1.
[Google Scholar]
|
| 17. |
Padmavathy L, Lakshmana RL, Ethirajan N, Krishnaswamy B, Manohar U. Epidermodysplasia verruciformis with Hansen's disease, Herpes simplex labialis and multiple eccrine hidradenoma. Indian J Dermatol 2009;54:S53-6.
[Google Scholar]
|
| 18. |
Gόl U, Kiliη A, Gφnόl M, Cakmak SK, Bayis SS. Clinical aspects of epidermodysplasia verruciformis and review of the literature. Int J Dermatol 2007;46:1069-72.
[Google Scholar]
|
| 19. |
Cortιs-Franco R, Tyring SK, Vega E, Payne D, Granados J, Dominguez-Soto L. Divergent clinical course of epidermodysplasia verruciformis in siblings. Int J Dermatol 1997;36:442-5.
[Google Scholar]
|
| 20. |
Jablonska S, Majewski S. Skin autograft in epidermodysplasia verruciformis: human papilloma virus associated cutaneous changes need over 20 years for malignant conversion. Cancer Res 1997;57:4214-6.
[Google Scholar]
|
| 21. |
Ortak T, Uysal AC, Alagoz YM, Orbay ZH, Sensoz OE. Epidermodysplasia verruciformis: an unusual presentation. Dermatol Surg 2006;32:302-6.
[Google Scholar]
|
| 22. |
Ekeowa-Anderson AL, Harwood CA, Perrett CM, Sahota A, Annan H, Ran H, et al. Vulvul intraepithelial neoplasia and periungual Bowen's disease concordant for mucosal (HPV-34) and epidermodysplasia verruciformis (HPV-21) human papilloma types. Clin Exp Dermatol 2007;32:304-7.
[Google Scholar]
|
| 23. |
Kowalzick L, Mensing H. Failure of eteretinate in epidermodysplasia verruciformis. Dermatologica 1986;173:75-8.
[Google Scholar]
|
| 24. |
Lutzner MA, Blanchet-Bardon C. Epidermodysplasia verruciformis. Curr Probl Derm 1985;13:164-85.
[Google Scholar]
|
| 25. |
Vu J, Wallace GR, Singh R, Diwan H, Prieto V, Rady P, et al. Combined variable immunodeficiency syndrome associated with epidermodysplasia verruciformis. Am J Clin Dermatol 2007;8:307-10.
[Google Scholar]
|
| 26. |
Gόl ά, Soylu S, Yavuzer R. Epidermodysplasia verruciformis associated with IgM deficiency. Indian J Dermatol Venereol Leprol 2007;73:420-2.
[Google Scholar]
|
| 27. |
Aochi S, Nakanishi G, Suzuki N, Setsu N, Suzuki D, Aya K, et al. A novel homozygous mutation of the EVER1/TMC6 gene in a Japanese patient with epidermodysplasia verruciformis. Br J Dermatol 2007;157:1265-6.
[Google Scholar]
|
| 28. |
Androphy EJ, Dvoretzky I, Lowy DR. X-linked inheritance of epidermodysplasia verruciformis. Arch Dermatol 1985;121:864-8.
[Google Scholar]
|
Fulltext Views
8,051
PDF downloads
3,545





