Translate this page into:
Eflornithine
Correspondence Address:
Kruti S Jobanputra
Department of Dermatology, K. J. Somaiya Medical College and Hospital, Mumbai, Maharashtra
India
| How to cite this article: Jobanputra KS, Rajpal AV, Nagpur N G. Eflornithine. Indian J Dermatol Venereol Leprol 2007;73:365-366 |

 |
| Figure 1: The polyamine pathway5 |
 |
| Figure 1: The polyamine pathway5 |
Introduction
Growth of coarse terminal hair in a male pattern in a woman is termed as hirsutism. In the general population, about 5% of women of reproductive age complain of hirsutism and it causes a lot of psychological stress affecting quality of life.
On the Ferriman Gallwey scale, hirsutism is indicated by a score of 8 or more. [1] Several methods like shaving, epilation, depilation, laser, electrolysis, and hormonal correction are available. Recently, a novel topical preparation, eflornithine has become available for the treatment of hirsutism. [2]
Eflornithine was synthesized as an anti-cancer drug in 1970 [3] and was later used intravenously in the treatment of African sleeping sickness. [4] Interestingly hair loss was observed as an adverse effect of this treatment. [5] Eflornithine hydrochloride cream (13.9%) is the first topical preparation approved by the FDA in August 2000 for reduction of facial hirsutism in women. [6]
Pharmacokinetics [7]
Eflornithine is also known as difluoromethylornithine or DFMO. Less than 1% of the topically applied eflornithine is absorbed. After four days of twice-daily application of the cream, steady-state plasma eflornithine concentrations were reached. Eflornithine is excreted unchanged in urine.
Mechanism of Action
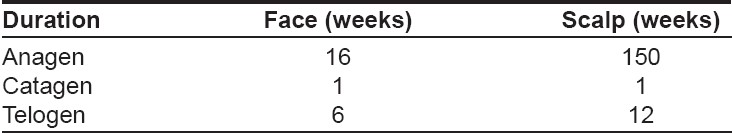
The pattern of hair growth is rhythmic and cyclical and consists of three phases i.e., anagen, catagen and telogen [Table - 1].
Hair growth can be slowed down in three ways: (1) by reducing the length of anagen phase; (2) by delaying the onset of anagen following the telogen phase; (3) by prolonging the telogen phase. Out of these three ways, eflornithine reduces the length of anagen phase. [6]
Ornithine decarboxylase which is an important enzyme for hair growth is irreversibly inhibited by eflornithine [Figure - 1]. Cell growth and differentiation is regulated by putrescine and other polyamines. The conversion of ornithine (which plays a vital role in the urea cycle) to putrescine is controlled by ornithine decarboxylase. Thus, rate of cell growth is reduced. [8]
Indications and Uses
Eflornithine is indicated for the reduction of facial hair growth in women irrespective of the cause of this hair growth.
Eflornithine doesn′t remove the excess hair but it causes slowing of excessive hair growth. [9] The hair volume is lessened making the hair finer and less apparent. [10] The frequency of hair removal by other means is reduced by eflornithine and it can be used as an adjuvant to other methods of hair removal (i.e. shaving, tweezing, waxing etc.). [4]
It is mainly used for the removal of facial hair, but it may be effective in other body areas as well. However, using this cream extensively in areas other than the face may increase the blood levels of the drug and thus the risk of adverse effects. [4],[11] Hence, the use of eflornithine has been restricted to the face and surrounding areas under the chin.
An improvement may be seen in 4 to 8 weeks of treatment or longer in some patients. It is recommended that the treatment be stopped after 6 months if no visible improvement.
After the treatment is stopped with eflornithine, improvement in hair appearance will be lost in about 8 weeks. [4] White or vellus hair which fail to respond to laser treatment can be treated with eflornithine. [3] Laser treatment given with eflornithine is more efficacious than laser treatment alone. [10]
Dosage and Administration [4]
A thin layer of eflornithine hydrochloride cream is applied to affected areas twice daily, at least 8 hours apart. The cream should be rubbed in thoroughly and the treated area should not be washed for at least 4 hours. The other hair removal methods that the patient is using should be continued with the use of eflornithine. Cosmetics or sunscreens can be applied after the cream has dried.
Adverse Effects [7]
Treatment related skin adverse events that occurred in less than 1% of the subjects treated with eflornithine are: acne (21%), pseudofolliculitis barbae, stinging skin, headache, burning skin, dryness, pruritus, erythema, tingling of the skin, dyspepsia, irritation, rash, alopecia, dizziness, folliculitis, ingrown hair, facial edema, anorexia, nausea, asthenia, vertigo, cheilitis, bleeding, contact dermatitis, swelling of lips, herpes simplex, numbness and rosacea.
Adverse effects are mild in nature and usually resolve without medical treatment or stopping eflornithine hydrochloride. Eflornithine should not be used in patients sensitive to any ingredients of the preparation
Eflornithine cream thus provides a fairly effective and simple means of reducing unwanted facial hair in women in conjunction with other methods of hair removal. This contributes to a great extent in reducing the anxiety related to the growth of unwanted facial hair in women. Due to the safety, tolerability and ease of administration, eflornithine cream will go a long way in the management of female hirsutism but it has to be kept in mind that it has to be used indefinitely to prevent regrowth of hair.
| 1. |
Rosenfield R. Hirsutism. N Engl J Med 2005;353:2578-88.
[Google Scholar]
|
| 2. |
Shenenberger DW, Utecht L. Removal of unwanted facial hair. American Family Physician 2002;66:10.
[Google Scholar]
|
| 3. |
Shapiro J, Lui H. Treatments for unwanted facial hair. Skin Therapy Lett 2005- 2006;10:10
[Google Scholar]
|
| 4. |
Powell P, Lucas K. Vaniqa (eflornithine hydrochloride) New Drug Update 2002;8:3.
[Google Scholar]
|
| 5. |
Coyne P. The eflornithine story. J Am Acad Dermatol 2001;45:784-6
[Google Scholar]
|
| 6. |
Shapiro J, Lui H. Vaniqa-Eflornithine 13.9% cream. Skin Therapy Lett. 2001;6:7.
[Google Scholar]
|
| 7. |
VANIQA� (eflornithine hydrochloride) Cream, 13.9% For topical dermatological use only. Not for ophthalmic, oral or intravaginal use.1/18/2004.www.rxlist.com/cgi/generic/index.html.29/9/2006.
[Google Scholar]
|
| 8. |
Malhotra B, Noveck R, Behr D, Palmisano M. Percutaneous absorption and pharmacokinetics of eflornithine hydrochloride 13.9% cream in women with unwanted facial hair. J Clin Pharmacol 2001;41:972-978.
[Google Scholar]
|
| 9. |
Trueb R. Causes and management of hypertrichosis. Am J Clin Dermatol 2002;3:617-627
[Google Scholar]
|
| 10. |
Watts J. Understanding the causes and management of hirsutism. Nursing Times 2006;102:8.
[Google Scholar]
|
| 11. |
Azziz R. The evaluation and management of hirsutism. Clinical Gynecologic Series: An Expert's View 2003;101:995-1007.
[Google Scholar]
|
Fulltext Views
13,557
PDF downloads
2,972





