Translate this page into:
Study of sepsis in dermatology ward: A preliminary report
2 Department of Medicine, All India Institute of Medical Sciences, New Delhi, India
3 Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
V K Sharma
Department of Dermatology, All India Institute of Medical Sciences, New Delhi
India
| How to cite this article: Sharma V K, Asati D P, Khandpur S, Khilnani G C, Kapil A. Study of sepsis in dermatology ward: A preliminary report. Indian J Dermatol Venereol Leprol 2007;73:367 |
Abstract
Background: Sepsis is an important cause of morbidity and mortality in dermatology inpatients. Aims: To assess the frequency, etiology and outcome of sepsis in dermatology ward and to formulate appropriate antimicrobial regimens. Methods: All inpatients were assessed for sepsis and its risk factors. Results: Ten patients out of a total of 150 inpatients (6.6%) developed sepsis. The commonly cultured organisms from skin and blood were Staphylococcus spp. (n = 20 isolates) and gram-negative organisms (n = 28). Three (30%) patients (2 TEN, 1 dermatomyositis) died. Conclusion: Sepsis was found to be an important event in our ward patients, with Staphylococci predominating in the list of causative microorganisms.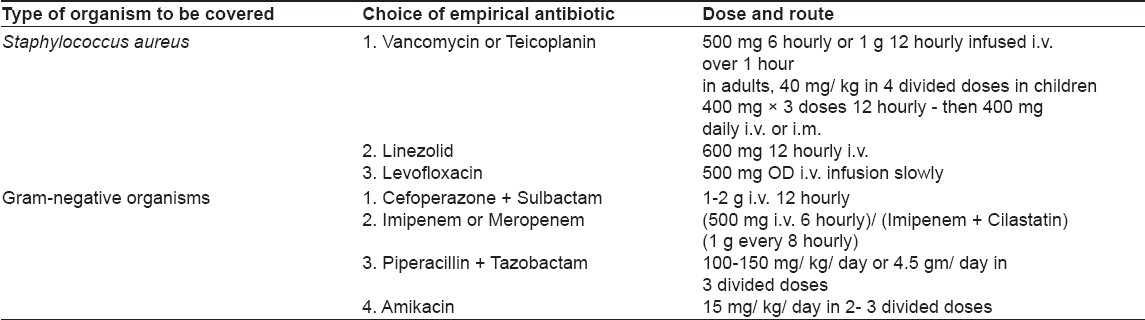




Sepsis is a very common and important cause of morbidity, mortality and economic loss in all hospitalized patients. [1],[2],[3],[4] The patients in dermatology ward, with large areas of their skin denuded and thus with severely compromised barrier and immune function of the skin, are especially susceptible to develop sepsis. The risk of sepsis is further accentuated by the use of steroids and other immunosuppressive/ cytotoxic agents, which are often given in high doses and for prolonged periods. [5] The mortality in dermatology ward can predominantly be ascribed to it directly or indirectly. [6] There is paucity of data regarding the epidemiological and etiological profile of sepsis in dermatology ward patients. A preliminary prospective study was carried out to assess the frequency, etiology and outcome of sepsis in dermatology ward and to formulate appropriate antimicrobial regimens.
Methods
During the 3-month study period, from November 2004 to February 2005, there were 10 patients of sepsis out of the 150 patients (6.6%) admitted to the Dermatology ward. Sepsis was defined by fulfillment of the systemic inflammatory response syndrome (SIRS) criteria - presence of two or more of the following features: [7]
a. Fever {oral temperature> 38°C} or hypothermia {< 36°C}
b. Tachypnea {>20 breaths per min} or PaCO 2 lower than 32 torr
c. Tachycardia {>90 beats per min}
d. Leukocytosis {>12,000/ µL }, leukopenia {< 4,000/ µL} or ≥10% ′band cells′ {immature neutrophils}
plus
Clinical or bacteriological evidence of presence of microorganisms as suggested by abscess, crusting, pyoderma or other evident focus of infection, clinical or radiological evidence of pneumonia, positive blood culture or any other relevant positive cultures like urine, sputum, etc.
A complete clinical assessment including detailed history, risk factor assessment and dermatological and systemic examination was undertaken. The patients were thoroughly investigated for any focus of infection and type(s) of organism(s) responsible for sepsis. Investigations included hemogram, peripheral smear (especially for band cells), ESR, liver and renal function tests, serum electrolytes, fasting blood sugar, chest X-ray and ECG. Samples for blood culture, skin swab or pus and other cultures (as relevant in each patient, e.g., sputum and urine culture) were taken with full aseptic precautions, and sensitivity pattern was tested. Antimicrobial sensitivity was performed on Muller-Hington agar (Hi-media India) by the standard disk diffusion method recommended by the National Committee for Clinical Laboratory Standards (NCCLS).
An episode of clinically significant bacteremia was defined as the isolation of one or more microorganisms (bacteria or fungus) from blood cultures associated with presence of SIRS. [8]
One positive blood culture was considered sufficient for commonly accepted pathogens. However, for Coagulase-negative Staphylococci or other skin contaminants, two or more consecutive positive blood cultures with identical susceptibility profiles in both the cultures were required. Nosocomial bacteremia was defined as bacteremia not present at the time of hospital admission but developing after 48 h or later.
Descriptive statistics, i.e., frequency distribution and percentages, were calculated for categorical variables, and mean and standard deviation were calculated for the continuous variables.
Results
A total of 150 patients were admitted during the study period. These included vesicobullous diseases (n = 45), systemic sclerosis (n = 32), erythroderma (n = 8) and severe drug reactions (n = 5). Of these, 15 (10%) patients showed features of SIRS. The criteria for sepsis were met in 10 patients (6.67%). Sepsis cases included vesicobullous disorders (n = 4), including pemphigus vulgaris (n = 3) and pemphigus foliaceus (n = 1), erythroderma (n = 3), TEN (n = 2) and dermatomyositis (n = 1) patients. Sepsis occurred in 3/ 37 (8.11%) patients of pemphigus vulgaris, 3/ 8 (37.5%) patients of erythroderma and 2/ 2 (100%) patients of TEN.
Their age ranged from 6-55 years (mean - 39.2 ± 15.3 years) and body surface area involvement from 5-100% (mean - 62 ± 40%) with more than 30% involvement in seven cases. The duration of dermatoses varied from 1 day to 18 years. In acute dermatoses, it varied from 24 h to 14 days (mean - 7.5 days, n = 2); while in chronic dermatoses, it ranged from 3 months to 18 years (mean - 62.5 ± 69.7 months).
There was history of prior hospitalization, within the last 15 days, in two patients; systemic illnesses like diabetes in three patients; and systemic steroid intake (>40 mg/ day for more than 1 week or> 20 mg/ day for more than 2 weeks) in seven patients. Four patients had received dexamethasone or dexamethasone-cyclophosphamide (DCP) pulse, while two patients were on other immunosuppressives (methotrexate and daily cyclophosphamide, in addition to pulse therapy).
DCP was administered to patients with pemphigus, systemic sclerosis or dermatomyositis, according to their clinical requirements. In the study group, two pemphigus vulgaris patients and one dermatomyositis patient received the pulse therapy. Analysis of a number of other admitted patients (without sepsis) receiving pulse therapy was not done as it was not a part of the study.
All patients were on intravenous cannula in the ward - three having urinary catheters, two on central venous line and three on artificial ventilation.
One patient had community-acquired bacteremia; while in the others, it was nosocomial in origin. In half of the sepsis patients, skin was the source of infection, as denoted by similar isolate in blood and pus or skin swab cultures. In one patient each, the lower respiratory tract and the urinary tract were the routes of sepsis, as suggested by a similar isolate in blood and tracheal aspirate or urine culture. The duration of stay was significantly prolonged in sepsis patients (41.5 ±30.4 days) as compared to nonsepsis patients (17.76 ± 17.60 days).
Culture and sensitivity pattern
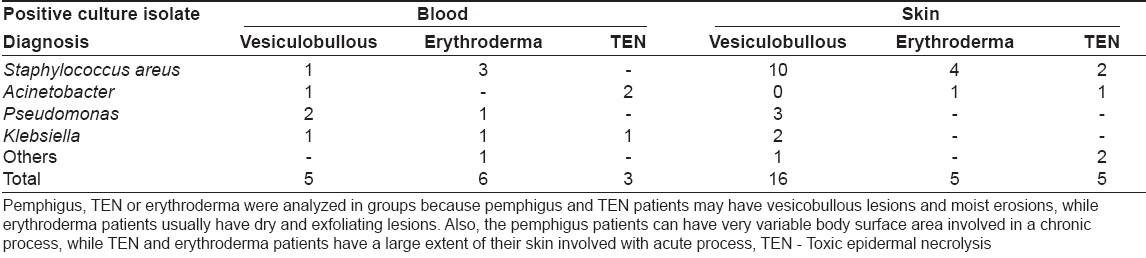
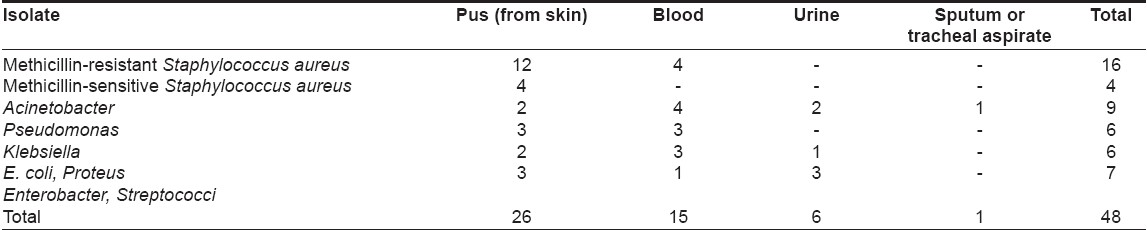
A total of 48 positive cultures were obtained from blood, pus, urine or respiratory tract; the organisms isolated are shown in [Table - 1]. The commonly cultured organisms from skin and blood were Staphylococcus spp . (n = 20 isolates; methicillin-resistant S taphylococcus aureus (MRSA) = 16, methicillin-sensitive Staphylococcus aureus (MSSA) = 4), followed by Acinetobacter spp. (9 isolates). Pseudomonas aeruginosa and Klebsiella pneumoniae were also grown in significant number of cultures (6 isolates each). In vesiculobullous patients, Staphylococcus aureus was the predominant organism in both blood and skin, while Acinetobacter spp . was the main organism grown in TEN patients [Table - 2]. Pseudomonas aeruginosa and Klebsiella pneumoniae growth did not correlate with a specific dermatosis.
Only one bacteremic episode due to Acinetobacter spp . , which was grown three times in a TEN patient, was community acquired, while 14 such episodes in nine patients were hospital acquired. Six pus isolates (2 MRSA , 1 MSSA , 2 E. coli and 1 Enterobacter spp . ) and one urine culture isolate ( E. coli ) originated in the community. Twenty isolates (10 MRSA , 3 MSSA and 7 gram-negative organisms) had nosocomial origin.
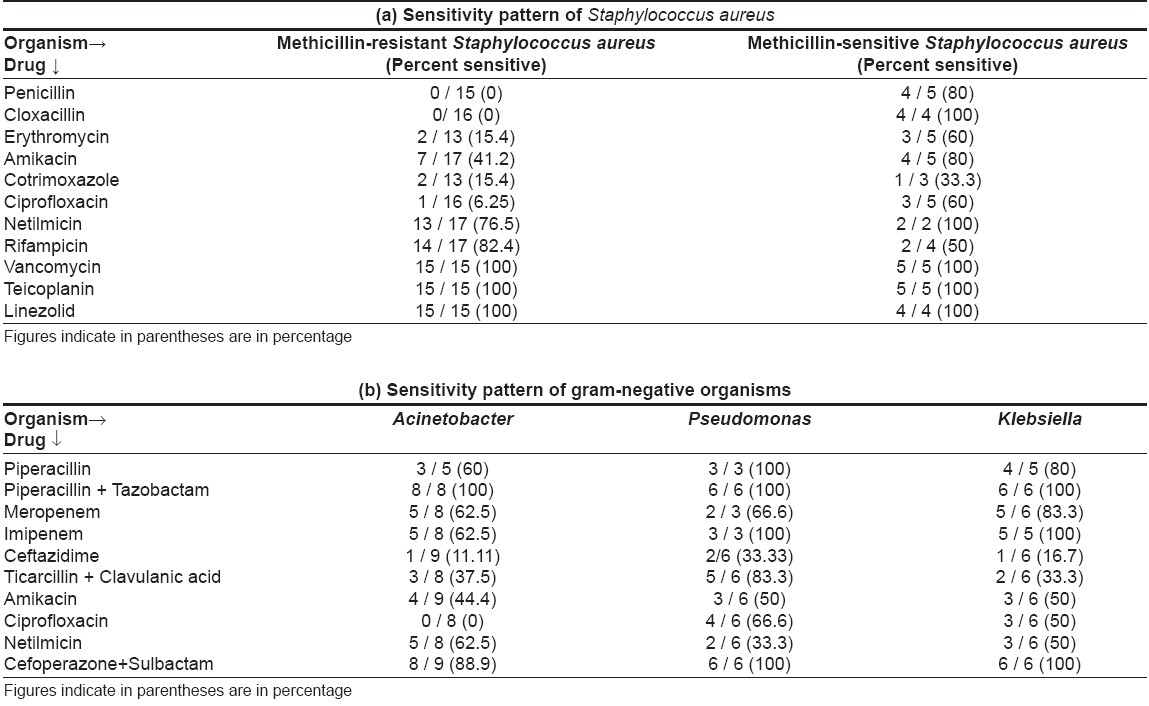
On sensitivity testing [Table - 3], Staphylococcus aureus was found sensitive to vancomycin and linezolid in all cases. Netilmicin covered a high percentage of isolates (76.5% for MRSA and 100% for MSSA ); amikacin was good for MSSA (100% sensitive) but not for MRSA (41.2% sensitive). Ciprofloxacin, co-trimoxazole, erythromycin, penicillin and cloxacillin showed poor sensitivity for Staphylococcus aureus .
Gram-negative bacteria were most sensitive to a combination of piperacillin + tazobactam (100%), followed by cefoperazone + sulbactam (88.9-100%), imipenem (62.5-100%) and meropenem (62.5-83.3%). Amikacin, ciprofloxacin, piperacillin, netilmicin, ceftazidime, ticarcillin + clavulanic acid combination showed low sensitivity. Three sepsis patients (30%), including two of TEN and one of dermatomyositis, died.
Discussion
Sepsis is defined as a microbial phenomenon characterized by inflammatory response to the presence of microorganisms or the invasion of normally sterile host tissue by these organisms. Inflammatory response is defined by the presence of two or more SIRS criteria cited above. [7] SIRS criteria exhibit a sensitivity of 69%, specificity of 35%, positive predictive value (PPV) of 90%, negative predictive value (NPV) of 12% and positive likelihood ratio (LR) of 1.06 in diagnosing sepsis. It was concluded that the finding of two or more SIRS criteria was of little usefulness for diagnosis of infection. [8] So, in the present study, sepsis was defined by presence of SIRS criteria along with evidence or strong suspicion of infection. This improved the specificity of the diagnostic criteria.
Several studies have been published in the literature relating to the problem of sepsis and its consequences in ICUs and other nondermatological settings. [1],[4],[9],[10],[11] However, there is paucity of such reports from the dermatology wards.
Zhang, in a study of 1,826 hospitalized patients, reported the highest incidence of nosocomial infections in dermatology ward (19.8%) compared to 13.1% in overall hospitalized patients. [12] In a small study by Nair et al. evaluating the cause of death in pemphigus and TEN patients, sepsis was found to be one of the leading causes of death in dermatology inpatients. [13]
The commonly cultured organisms in our study were Staphylococcus aureus, followed by Acinetobacter, pseudomonas and Klebsiella spp . Staphylococcus has been shown to be the commonest pathogen in bloodstream or soft tissue infections, in both Indian and international studies. [9],[14] Kanwar et al. found staphylococcal septicemia to be the leading cause of death (4 out of 10 deaths) in pemphigus patients. [10] Acinetobacter was the commonest gram-negative organism isolated in critically ill patients by Jang et al. [11]
In our patients, 14 bacteremic episodes were hospital acquired as compared to only 1 community-acquired bacteremia. Acinetobacter and MRSA were the predominant organisms isolated in community-onset infections. They may be an indicator of increase in nonjudicious use of antibiotics in the general population.
Sensitivity pattern in our study corroborates with another recent study conducted in the Department of Microbiology of our institute. [14] It also reported good sensitivity of gram-negative organisms (Pseudomonas, Acinetobacter and Klebsiella spp .) to piperacillin + tazobactam (94.4%), with poor sensitivity to piperacillin alone (35.0%), ceftazidime (33.3%), amikacin (34.8%), netilmicin (48.1%) and ciprofloxacin (40.2%). Gram-positive organisms ( S. aureus ) also showed high sensitivity to vancomycin (100%), rifampicin (81.21%) (linezolid was not tested) and poor sensitivity to ciprofloxacin (47.68% resistance) and ampicillin (50.99% resistance).
In our study, 6.67% of the inpatients during the 3-month study period developed sepsis, of which 30% died. This study validates the requirement of attention into this important aspect of management in dermatology wards. This mortality rate (3 out of 10 patients, 30% mortality) is comparable to the 30-35% mortality rate reported in other studies. [15],[16]
Our study is an attempt to understand the organisms responsible for sepsis in our dermatology ward and their current sensitivity patterns. Empirical antibiotic guidelines are proposed for adequate coverage of sepsis patients before culture reports become available [Table - 4]:
- Same class of antibiotics should not be used in all ward patients at the same time; otherwise, development of resistance will be faster.
- Affordability and availability factors should always be kept in mind.
- Empirical coverage in a sepsis patient should include one antibiotic having antistaphylococcal activity and one sensitive against gram-negative bacteria.
- Treatment should always be individualized on the basis of clinical assessment. Sometimes, it may not be necessary to give multiple antibiotics, especially if a single antibiotic can cover the whole spectrum of suspected infections.
Along with the systemic antibiotics, Condy′s compresses and hygienic bath were advised to the patients with crusted or oozy lesions.
A larger study will provide more relevant and accurate data regarding the etiology and management of sepsis in a dermatology ward; such a study is being planned.
| 1. |
Pittet D, Tarara, D, Richard P. Nosocomial bloodstream infection in critically in patients. Excess length of stay, extra costs and attributable mortality. JAMA 1994;271:1598-601.
[Google Scholar]
|
| 2. |
Doebbelling BN, Wenzel RP. The direct costs of universal precautions in a teaching hospital. JAMA 1994;264:2083-7.
[Google Scholar]
|
| 3. |
Riedmann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest 2003;112:460-7.
[Google Scholar]
|
| 4. |
Garrouste-Orgeas M, Chevret S, Mainardi JL, Timsit JF, Misset B, Carlet J. A one year prospective study of nosocomial bacteremia in ICU and non ICU patients and its impact on patient outcome. J Hosp Infect 2000;44:206-13.
[Google Scholar]
|
| 5. |
Pasricha JS. Pulse therapy in pemphigus and other diseases. 2 nd ed. New Delhi: Pulse Therapy and Pemphigus Foundation; p. 19, 30,31.
[Google Scholar]
|
| 6. |
Ahmed AR, Moy R. Death in pemphigus. J Am Acad Dermatol 1982;7:221-8.
[Google Scholar]
|
| 7. |
American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864-74.
[Google Scholar]
|
| 8. |
Jaimes F, Garcιs J, Cuervo J, Ramνrez F, Ramνrez J, Vargas A, et al . The systemic inflammatory response syndrome (SIRS) to identify infected patients in the emergency room. Intensive Care Med 2003;29:1368-71.
[Google Scholar]
|
| 9. |
Vallιs J, Le�n C, Alvarez-Lerma F. Nosocomial bacteremia in critically ill patients: A multicenter study evaluating epidemiology and prognosis. Spanish Collaborative Group for Infections in Intensive Care Units of Sociedad Espanola de Medicina Intensiva y Unidades Coronarias (SEMIUC). Clin Infect Dis1997;24:387-95.s
[Google Scholar]
|
| 10. |
Kanwar AJ, Dhar S. Factors responsible for death in patients with pemphigus. J Dermatol 1994;21:655-9.
[Google Scholar]
|
| 11. |
Jang TN, Kuo BI, Shen SH, Fung CP, Lee SH, Yang TL, et al . Nosocomial gram-negative bacteremia in critically ill patients: Epidemiological characteristics and prognostic factors in 147 episodes. J Formos Med Assoc 1999;98:465-73.
[Google Scholar]
|
| 12. |
Zang Y. A two-year prospective survey on nosocomial infections. Zhonghua Yi Xue Za Zhi 1991;71:253-6.
[Google Scholar]
|
| 13. |
Nair PS, Moorthy PK, Yogiragan K. A study of mortality in dermatology. Indian J Dermatol Venereol Leprol 2005;71:23-5.
[Google Scholar]
|
| 14. |
Mohanty S, Kapil A, Dhawan B, Das BK. Bacteriological and antimicrobial susceptibility profile of soft tissue infections from northern India. Indian J Med Sci 2004;58:10-15.
[Google Scholar]
|
| 15. |
Lark RL, Chenoweth C, Saint S, Zemencuk JK, Lipsky BA, Plorde JJ. Four year prospective evaluation of nosocomial bacteremia: Epidemiology, microbiology and outcome. Diag Microbiol Infect Dis 2000;38:131-40.
[Google Scholar]
|
| 16. |
Pittet D, Wenzel RP. Secular trends in rates, mortality and contribution to total hospital deaths. Arch Intern Med 1995;155:1177-84.
[Google Scholar]
|
Fulltext Views
2,640
PDF downloads
2,816





