Translate this page into:
Skin scraping and a potassium hydroxide mount
2 Central Clinical Microbiology Laboratory, Thane, Maharashtra, India
Correspondence Address:
Sachin M Kurade
Department of Dermatology, 'O' Building, B.Y.L. Nair Hospital, Mumbai
India
| How to cite this article: Kurade SM, Amladi SA, Miskeen AK. Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol 2006;72:238-241 |
 |
 |
 |
 |
Diagnostic cytology involves various methods like aspiration cytology, imprint smears, skin scraping smear and Tzanck smear. In dermatology, a potassium hydroxide (KOH) mount of a skin scraping is a common procedure performed to demonstrate the evidence of fungal infection in skin, hairs and nails. It can be done on an outpatient basis and the results are available within 1-2 h.[1]
In experienced hands, a potassium hydroxide mount is one of the most useful procedures in medical mycology. It has been adjudged more reliable than culture for demonstration of dermatophytes.[2]
The standard method involves the following steps:
Scraping
This is a procedure in which the infected lesions are scraped to confirm the presence of fungal infection by microscopic examination. The lesions should be thoroughly cleaned free of any debris or medication by using alcohol or by washing. Separate potassium hydroxide mounts should be prepared from specimens collected from different sites. Application of little distilled water to the site usually enables easy sampling of the scales. The skin above the site should be pulled up with one hand and the scalpel edge moved across the edge of the lesion with the other.
In hairy areas like scalp and beard, scraping along the edges, besides epilating short hair stubs and crusts, helps obtain a better yield.
In nail involvement, scraping the affected sites at a considerable depth is advisable. Scooping out the deeper keratinous matrix and mounting it in 20% potassium hydroxide yields better results. Nail clippings and avulsed whole nails can be held with a pair of clean blunt forceps and the undersurface or dystrophic edges scraped off, for microscopy.
Potassium hydroxide mount
A scraping of the skin is usually taken with a pre-flamed blunt scalpel from the edge of the lesion. Scrapings may be collected in a black paper or directly on to the slide. Potassium hydroxide 10% is added to the collected material, covered by a cover slip made of fragile glass and gently preheated before examining for fungi.
Microscopic examination
This is a direct microscopic examination of the above specimen to detect fungal spores or hyphae. Initial examination is with low power magnification (x10) and low intensity of light with lowering of the condenser. Later, for a higher magnification (x40), the condenser should be higher for better illumination to study the morphology of the fungus. Fungal spores vary from 2-10 mm in diameter. Sometimes the lines of juncture of normal epidermal cells dissolve into branching network and are easily mistaken for a fungal structure. This is called ′mosaic fungus′ and is the most common artifact. Other artifacts that may mimic fungal hyphae are cotton fibers and synthetic fibers.
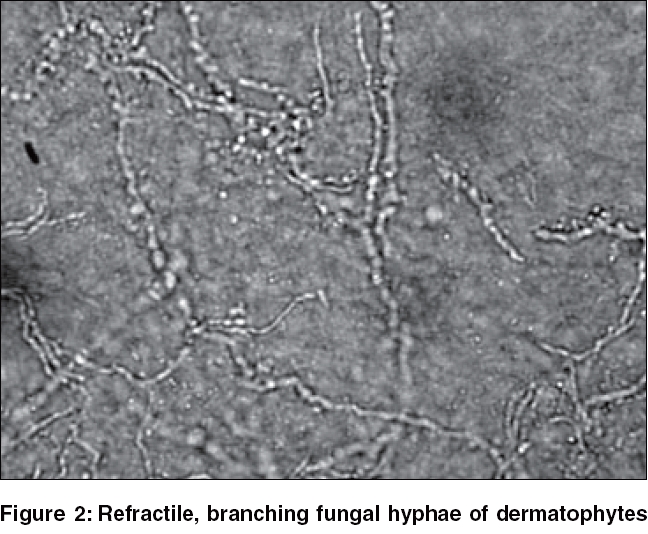
Key points· As far as possible, the potassium hydroxide solution should not be applied to the skin lesions, particularly on the face or on mucous membranes. · A cover-slip is applied with a little pressure to blot away the excess potassium hydroxide and make the preparation even when examining under the microscope. · To avoid distortion and loss of texture in fragile infected hair fragments, do not press the fresh cover-slip preparation of the hair mount. · For thick, hyperkeratotic specimens, leave the potassium hydroxide preparation for ′digestion′ and ′clearing′ for ½ to 2 h. This clearing time for nails and hairs may extend to 24-48 h. · Should the mount dry, air pockets get formed under the cover-slip; add more potassium hydroxide solution for reexamination. · Note the refractile, long, smooth, undulating, branching and septate hyphal filaments with or without arthroconidiospores in dermatophyte involvement. · The hyphal filaments seen by microscopy in nondermatophytic mold involvement of the nail, especially of the great toes, tend to be irregular, vesiculated, tortuous or pigmented. · Note the dozens of hyaline, oval, budding (blastoconidiating) yeast cell forms with or without pseudo-hyphal filaments in candidal involvement. · Thick-walled spherical yeast in clusters, often with short filaments resembling ′banana and grapes′ or ′spaghetti and meatballs,′ are characteristic of Malassezia furfur in pityriasis versicolor scales.
Diagnostic uses of KOH
Dermatophytic and candidial infection of skin[5],[6],[8]
A potassium hydroxide examination of a smear from scales or erosions from lesions shows hyphae [Figure - 1][Figure - 2] or pseudohyphae with yeasts.
Dermatophytic infection of hairs (including black piedra)[6]
Infected hair appearing as broken stubs are best for examination. They can be removed with forceps without undue trauma or collected by gentle rubbing with a moist gauze pad or toothbrush. Dermatophyte infection of hair will show spores, either within the hair shaft (endothrix) or outside the hair shaft (ectothrix), on a potassium hydroxide mount. In ectothrix, spores are seen grouped and attached outside the hair cortex. However, in endothrix, they are seen not only packed inside the hair cortex but also may be seen outside the hair cortex and may be even associated with dystrophy of hair shaft.
Dermatophytic and candidial infection of nails[6],[7]
A potassium hydroxide examination of scrapings from the nail plate shows hyphae or pseudohyphae with blastoconidiating yeast cells.
Pityriasis versicolor[7]
A potassium hydroxide examination of scrapings from lesions shows a ′banana and grapes′ or ′spaghetti and meatballs′ appearance.
Vaginosis[8]
Bacterial vaginosis is a common cause of vaginitis in women in the childbearing age who are sexually active. The vaginal fluid obtained on removal of the speculum should be tested for pH and a few drops of 10% potassium hydroxide added to the discharge on a glass slide and sniffed for detection of a fishy odor. Light microscopy of immediate wet mounts can be done to identify ′clue cells′ of Gardernella.
Blue neck syndrome[9]
This is a common condition in Northern Kerala. The affected skin has a dull dry matt surface with a characteristic bluish-black color, with a distinctive pigmentation of the surface and the skin folds clearly visible as nonpigmented grooves. Potassium hydroxide mounts of skin scrapings from the neck when examined under a microscope show nematode larvae. Tinea nigra[10]
A potassium hydroxide mount of the scrapings shows typical fungal elements which on culture grow Exophiala werneckii .
Demonstration of mites
Mites like Demodex folliculorum (in rosacea-like lesions) and Sarcoptes scabiei (in hyperkeratotic or crusted scabies) can be demonstrated in a 10% potassium hydroxide mount from skin lesions.
A high index of clinical suspicion, very good corneal scraping (deep scraping) using a no.15 blade on a Bard Parker handle and thorough and accurate microscopic examination of direct smears yield 100% sensitivity of 10% potassium hydroxide smear. Specimens of debrided epithelium from corneal ulcers appearing as whitish flakes are directly mounted in 10% potassium hydroxide and examined for molds ( Aspergillus , Fusarium ), yeasts or Nocardia spp.
Demonstration of fungi from cutaneous lesions of deep fungal infections
In cutaneous cryptococcosis, a drop of serum or exudate from a lesion mounted on a slide with a few drops of 10% potassium hydroxide and India ink will demonstrate the capsulated forms of Cryptococcus .[13] In chromoblastomycosis, a potassium hydroxide mount of skin scraping from the surface of lesions shows sclerotic or muriform fungal cells. In blastomycosis, a potassium hydroxide mount of pus, skin scraping or sputum shows round, refractile spherical cells with broad-based buds.[14]
Modifications of the standard method[15]
Cellophane tape method
A 5 cm long and 2 cm wide scotch tape (transparent cellophane tape) can be applied over the affected site, pressed firmly and removed. The tape is then stuck on the surface of a glass slide and sent to the laboratory, where it is gently lifted and replaced after placing 3 to 4 drops of 10% potassium hydroxide solution. The undersurface of the slide is warmed gently and examined under microscope. In addition, the tape can be folded after stripping the horny layer by bringing the adhesive surfaces together. The folded tape can then be sent to the laboratory where it is reopened and mounted with potassium hydroxide as described above. A tiny bit of paper is attached to each end to enable easy separation of the folded tape. This modified method has the advantage of cellophane replacing the cover-slip and hence of easy transportation.
Parker′s ink method
Parker′s ink can be added to potassium hydroxide. Parker′s ink stains the fungal wall blue and is thus easy to recognize.
Eosin 1% method
Eosin 1% can be added to potassium hydroxide to stain the keratin. It lends a pinkish background, while the fungal elements remain unstained.
Modified Parker′s ink and 1% eosin method
Add 1% eosin to Parker′s ink in 2:1 proportion by volume to prepare modified Parker′s ink. The mixture is painted over the affected site and allowed to dry. Then reapply the cellophane tape, gently press it, remove it, stick it over a glass slide and view. The background stains pink due to eosin, while the fungal elements stain blue due to ink. No heating is required. Eosin and Parker′s ink can be combined to offer a simple method to demonstrate fungi. The specimen is also easy to transport and does not need warming.
Calcofluor white
This is a colorless dye, a fluorochrome stain, for rapid detection of fungi in wet mounts, smears and tissue sections, especially in scrapings from skin and mucous membrane. Viewed under ultraviolet light, fungal structures display a brilliant apple-green or a ghostly blue-white color.
Pros and cons
KOH mount is an easy-to-perform rapid, inexpensive test that is simple to perform. Although it requires minimum of infrastructure, it takes a little experience to interpret the smears. However, it must be remembered that the test is not highly specific in onychomycosis or dermatophytosis and can′t be specifically used for monitoring of treatment in these conditions. There is also some concern about dissemination of infection to nearby areas during the test and if care is not taken, it may be painful to patients.
A potassium hydroxide mount can prove to be an important, yet simple, office investigation in dermatology that gives diagnostic information.
| 1. |
Gupta L, Singhi MK. Tzanck smear: A useful diagnostic tool. Indian J Dermatol Venerol Leprol 2005;71:295-9.
[Google Scholar]
|
| 2. |
Rippons JW. Dermatophytoses and Dermatomycosis. In : Rippons JW, editor. Medical Mycology, the Pathogenic fungi and the Pathogenic Actinomycetes, 2nd ed. W. B. Saunders: Philadelphia; 1983. p. 213.
[Google Scholar]
|
| 3. |
Thirumurthy M, Sethuraman G, Srinivas CR. KOH mount for superficial fungal infections using cellophane tape: Comparison with standard technique. Indian J Dermatol Venereol Leprol 2002;68:136-6.
[Google Scholar]
|
| 4. |
Oberoi CM, Miskeen AK. Superficial fungal infections. In: Valia RG, Valia AR, editors. IADVL Textbook and Atlas of Dermatology, 1st ed. Bhalani Publishing House: Mumbai, 1994 p. 173-212.
[Google Scholar]
|
| 5. |
Diseases Resulting from Fungi and Yeasts. In: James WD, Berger TG, Elston DM, editors. Andrews' Diseases of skin; Clinical Dermatology: 10th ed. W. B. Saunders: Philadelphia; 2005. p. 304.
[Google Scholar]
|
| 6. |
Hay RJ, Moore MK. Mycology. In : Champion RH, Burton JL, Burns DA. Breathnach SM, editors. Rook/ Wilkinson/ Ebling Textbook of dermatology. 7th ed. Oxford: Blackwell Science; 1998. p. 31.40-31.49 and 31.72-3.
[Google Scholar]
|
| 7. |
Klenk AS, Martin AG, Heffernan MP. Yeast infections: Candidiasis, Pityriasis (Tinea) Versicolor, In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Textbook of Fitzpatrick's Dermatology in General Medicine 6th ed. McGraw Hill: New York; 2003. p. 2016.
[Google Scholar]
|
| 8. |
Rao PS, Devi S, Shriyan A, Rajaram M, Jagdishchandra K. Diagnosis of bacterial vaginosis in a rural setup: Comparison of clinical algorithm, smear scoring and culture by semiquantitative technique. Indian J Dermatol Venereol Leprol 2004;22:47-50.
[Google Scholar]
|
| 9. |
Sugathan P, Jayaram CP. The Blue Neck Syndrome: Nematode Larvae in Skin Scrapings. Indian J Dermatol Venereol Leprol 2000;66:182-4.
[Google Scholar]
|
| 10. |
Hemashettar BM, Patil CS, Siddaramappa B, Thammayya A. A Case of Tinea Nigra From South India. Indian J Dermatol Venereol Leprol 1985;51:164-6.
[Google Scholar]
|
| 11. |
Bharathi MJ, Ramakrishnan R, Vasu S, Meenakshi R, Palaniappan R. Epidemiological characteristics and laboratory diagnosis of fungal keratitis. A three-year study. Indian J Ophthalmol 2003;51:315-21.
[Google Scholar]
|
| 12. |
Sharma S, Athmanathan S. Diagnostic procedures in infectious keratitis. In: Nema HV, Nema N, editors. Diagnostic Procedures in Ophthalmology. Jaypee Brothers Medical Publishers: New Delhi; 2002. p. 232-53.
[Google Scholar]
|
| 13. |
Diseases Resulting from Fungi and Yeasts. In: William D James, Timothy G Berger, Dirk M. Elston, editors. Andrews' Diseases of skin, Clinical Dermatology 10th ed. W. B. Saunders: Philadelphia; 2005. p. 319.
[Google Scholar]
|
| 14. |
Hay RJ. Deep Fungal Infections. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Textbook of Fitzpatrick's Dermatology in General Medicine, 6th ed. McGraw Hill: New York; 2003. p. 2021-26.
[Google Scholar]
|
| 15. |
Thirumurthy M, Sethuraman G, Srinivas CR. Demonstration of fungus by using parker's India ink and eosin- a simple technique. Indian J Dermatol Venereol Leprol 2002;68:376.
[Google Scholar]
|
Fulltext Views
53,751
PDF downloads
6,603





