Translate this page into:
Pigmentary demarcation lines over the face
Correspondence Address:
V K Somani
Plot No. 17-A, Journalist A Colony, Jubilee Hills, Hyderabad, Andhra Pradesh
India
| How to cite this article: Somani V K, Razvi F, Sita VV. Pigmentary demarcation lines over the face. Indian J Dermatol Venereol Leprol 2004;70:336-341 |
Abstract
BACKGROUND: We have been observing that a significant proportion of our patients, especially females, have certain pigmentary demarcation lines (PDL) over the face. However, systematic studies of the subject are lacking. AIMS: We categorized the different clinical patterns of facial PDLs in the Indian subpopulation and assessed their prevalence in this study. METHODS: About 4000 consecutive patients, both males and females, attending our skin clinic were examined for the presence of any pigmentary demarcation lines on the face, from October 1998 to February 2000. RESULTS: Out of the study population of 4037 patients, 243 (6%) were found to have demarcation lines on the face. The demarcation lines were far more common in women (9%) than in men (0.75%). These lines could be classified into three patterns that we would like to label as F, G, H as PDLs A to E have already been described. CONCLUSIONS: Pigmentary demarcation lines are fairly common in the Indian population especially amongst the females. Hormonal influences could possibly explain the female preponderance. Aggregation of cases within families or among close relatives suggests a genetic background.



 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
INTRODUCTION
Pigmentation is generally perceived as an adaptation response of humans to environmental conditions, especially ultraviolet radiation.[1] However, there is considerable variation even in a single race. For example, the skin color of Caucasians ranges from fair to brown. The constitutive pigmentation also differs in a race, females having a lighter color than males. The distribution of melanin is also uneven in a given race, e.g. in Caucasians the upper thigh is the darkest and the lumbar, the lightest area. Racial pigmentation differs not because of differences in melanocyte number but because of several other reasons, like size of melanosomes, extent of melanization and the arrangement of melanosomes within keratinocytes. Some darkly pigmented areas are regarded as normal, such as the genital area, the elbows and the knees, the knuckles and in many, a mild degree of infraorbital pigmentation. In addition to this, pigmentary demarcation lines have been described in the skin of Africans and Japanese.[2]
Pigmentary demarcation lines, also known as Futcher′s or Voight′s lines, are physiological, abrupt transitions from deeper pigmented skin to lighter pigmented skin. Five naturally occurring PDLs, labeled A-E, have been described. Briefly, they appear as follows:
A - On the lateral aspect of the upper arm extending over the pectoral area,
B - On the posteromedial portion of the lower limb,
C - Medio-sternal line, a vertical hypopigmented line in the pre and parasternal area,
D - On the posteromedial area of the spine, and
E - Bilateral hypopigmented streaks, bands or lanceolate areas over the chest in the zone between the mid-third of the clavicle and the periareolar skin.
Pigmentary demarcation lines have been described before in the Indians over the face.[15] We studied patterns of pigmentary demarcation lines seen over the face in about 4000 patients visiting our clinic.
MATERIALS AND METHODS
The study population comprised 4037 patients who attended the skin out-patient department for various complaints from October 1998 to February 2000. Patients giving a history of using cosmetic creams were excluded from the study. Patients who gave a history of any drug intake in the last six months were also excluded as many patients could not remember the medications taken earlier for various problems. All those patients who came to the skin out-patient department specifically for any pigmentary anomaly or who gave a history of previous facial eruptions were also excluded to avoid confusion with other pigmentary disorders and post-inflammatory pigmentation.
Each patient was examined for any facial pigmentation and patients with pigmentary diseases like melasma, pigmented cosmetic dermatitis, seborrheic melanosis, photodermatosis, lichen planus, nevi, etc. were excluded. A detailed history was taken emphasizing upon the family history, exposure to the sun, usage of cosmetics/soaps, etc. Patients were asked about any drug intake and application of any topical medicaments. Associated pigmentary anomalies like macular amyloidosis, acanthosis nigricans, seborrhoeic melanosis and infraorbital pigmentation were noted. A history regarding occupational exposure to tar and related products was also elicited. After going through the history, the subjects were examined by all the authors after the out-patient hours for classifying the PDLs and to draw sketches.
After obtaining informed consent, representative biopsies were taken from patients of all types of PDLs to exclude other causes of pigmentation.
RESULTS
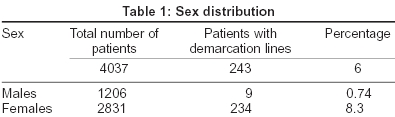
Out of the total study population of 4037 patients, 2831 were females and 1206 were males. The breakup of the number of patients presenting with facial pigmentary lines is given in [Table - 1].
The number of male patients with demarcation lines was negligible (0.74%) when compared to the number of females (8.3%). The pigmentary demarcation lines were of the following three types:
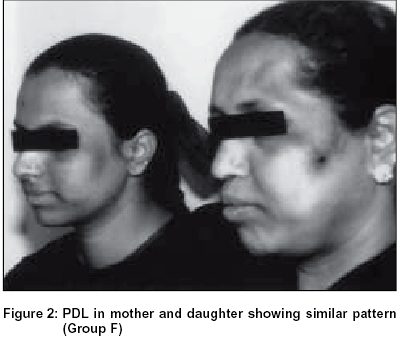
Group F: 4.56% of the total female patients surveyed presented with hyperpigmented patches that appeared ′V′ shaped or as an inverted cone on the lateral aspect of the face in the region between the malar prominence and the temple. They were invariably bilaterally symmetrical and of almost equal intensity on both the sides in a given patient. In about 20% of cases these patches merged with infraorbital pigmentation [Figure - 1], [Figure - 2], [Figure - 3].
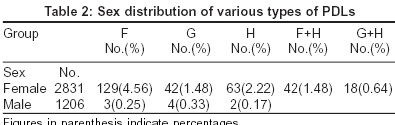
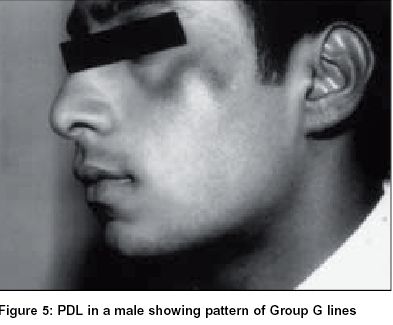
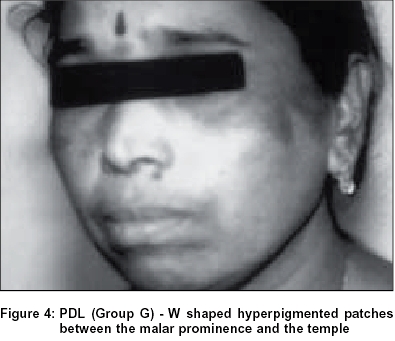
Group G:Of the study population, 1.48% of females had the pigmentary patch in the same location but had two inverted cones lying in close proximity, looking like the letter W, with a faint strip of normal pigmentation in between. Both patterns F and G showed evenly diffuse pigmentation with a rather well defined margin which was better appreciated from a little distance. There were no satellite lesions, speckling, increased hair or any textural change [Figure - 4], [Figure - 5].
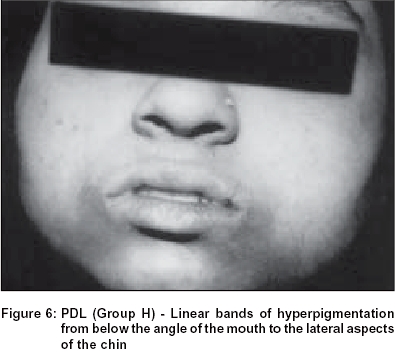
Group H: In 2.2% of the study population, we observed two symmetrical linear bands of hyperpigmentation extending from just below the angle of the mouth to the lateral aspects of the chin. In many women there was an additional band running just below and parallel to the lower lip, joining the two oro-mental bands [Figure - 6].
All the above patterns were seen more clearly in wheatish complexioned patients than in those with a very fair or dark complexion. In a fair percentage (36%) of people - included with PDLs of groups F and G we noticed para-oral bands (Group H) also. The pattern wise break-up of the pigmentary demarcation lines over the face in our study population is given in [Table - 2].
All the pigmentary demarcation lines first made their appearance around puberty and tended to persist throughout life. We had patients from 16 years to above 60 years in our study and on questioning gave consistently the history of appearance of the PDLs since around puberty. About 50% of the patients presented to the dermatology department with the complaint of pigmentation whereas an additional 25% were aware of the problem but did not complain of it. The rest of the patients were unaware of the pigmentation.
The histopathology was unremarkable except for increased melanization in 12 cases. Congo Red stain for amyloid was negative.
DISCUSSION
Skin pigmentation shows interesting variations in a single race or in an ethnic subpopulation. About 25% of black Africans show sharply demarcated pigmentary lines either at the anterolateral upper arm or posteromedial part of the lower limbs.[3],[4] Weary et al and Selmanowitz described hypopigmented lines, bands or macules over the anterior chest.[5],[6]
Selmanowitz and Krivo later concluded that there are five types of PDLs and that these occur in both black Africans and Japanese.[2] Similar pigmentary lines that later regressed were also described in Puerto Rican women on the lower limbs as a physiologic change in pregnancy.[7] A population survey that included both black and white patients showed the presence of pigmented lines in black female adults, black male adults and white female patients in 79%, 75% and 15% respectively.[8] A rare type of facial pigmentation pattern was described in three adult males, which was attributed to chemical photosensitisers or excessive sun exposure.[9]
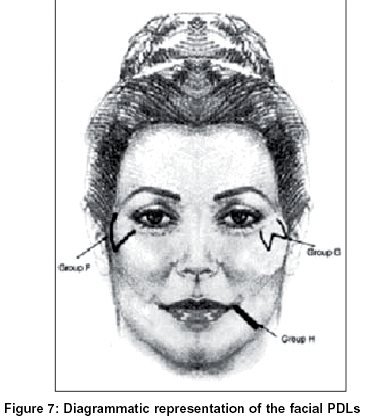
Three distinct patterns of PDLs emerge from the data presented in this study. To summarize, the patterns were as follows [Figure - 7]:
F - ′V′ shaped hyperpigmented lines between the malar prominence and the temple
G - ′W′ shaped hyperpigmented lines between the malar prominence and the temple
H - Linear bands of hyperpigmentation from the angle of the mouth to the lateral aspects of the chin.
The facial PDLs in our study appeared around puberty in contrast to their origin in early childhood in the case of the population survey[8] carried out earlier. PDLs A-E have been described on the trunk and limbs whereas patterns F-G were seen only on the face. Earlier studies for PDLs A-E have an equal or a significant number of male black patients[2],[8],[10] affected. On the other hand, we found that for lines F-G, females constituted the great majority of patients, with males accounting for a minuscule 1%. Facial PDLs were not associated with disease states[11] or emaciation and relaxation of the skin.[10] But, as observed by Vollum[12] for other PDLs, the facial PDLs also become more apparent with advancing age.
Facial PDLs can often be misdiagnosed as melasma, post-inflammatory pigmentation, nevus of Ota or Ito, or melanocytic nevus. Melasma occurs as a blotchy pigmentation over the malar areas just below the PDL F, and may involve other parts of the face like the nose, supra-orbital areas or upper lips. Dermal nevi often have a bluish or greenish black color and a scleral component.
In both Japanese and in African blacks, it has been proposed that the various PDLs are dominantly inherited.[5],[6],[13] Miura[14] opined that PDLs coincide with cutaneous nerve distribution. Group A PDL has been referred to as Voigt′s line[12],[13] but it is now considered as corresponding to one of Voigt′s line in the axial course.[2] Several linear dermatoses have been reported which correlated with PDLs giving rise to speculation about the marginal lines of cutaneous innervation and embryonic suture lines influencing the disease pattern. Malakar et al described PDL over face in the Indian population, which corresponds to our PDL ′F′, except that the pigment intensity on both sides of the face is usually the same in our series.[15]
Cutaneous mosaicism at the cellular level has been implicated in at least 15 different skin disorders.[16] Cutaneous mosaics belong to two categories: functional mosaics, resulting from X inactivation or lyonization, and genomic mosaics, caused by postzygotic autosomal mutations. Happle reviewed the pigmentary patterns associated with mosaicism and described 5 types of pigmentary patterns.[17],[18] In functional mosaicism, either the maternal or paternal X chromosome is lyonized randomly, but it remains the same for all the descendants of that cell. Because of this process the heterozygous state of various X-linked gene defects may lead to a cutaneous mosaic. Streaky or patchy pigmentation may be a clue to the presence of mosaicism. Lyonization of the paternal X chromosome in these cutaneous mosaic phenotypes could explain the familial aggregation and the female preponderance of these facial demarcation lines. However further studies in such phenotypes could provide an explanation for the occurrence of these PDLs.
Since this study was done in a hospital setting, another study across a wider cross-section of the population is also being undertaken. Facial patterns, especially types F and G, were seen regularly in other family members like sisters and mother (61%), pointing to a genetic predisposition [Table - 3]. Because of their similar location and morphological resemblance, type G line could represent a variant of type F line. Unfortunately our study population did not have any twins. Hormonal influence over pigmentary characteristics has been observed.[19] The preponderance of females exhibiting these lines could be related to sex hormones.
Patients suffering from facial PDLs often seek treatment. All the patients who came to us with the complaints (50% of the total) and 20% of the other patients who desired treatment (10% of the total) were put on Kligman regimen for 3 months along with a sunscreen. Strict avoidance of sunlight was advised. However, there was only a negligible change in the colour. Of these, 15 patients were treated with 6 sittings of glycolic acid peels at 3 weekly intervals starting with 35% and increasing gradually to 70%. There was about 10% reduction in skin pigmentation and after stopping the treatment the pigmentation came back.
To summarize, three patterns of pigmentary demarcation lines over the face, types F-H, are described in the Indian population. These patterns, which first make their appearance at or around puberty, may have a genetic predisposition and remain more or less unchanged throughout life. Their appearance is distinctive enough to be recognized as a fairly common variant of normal pigmentation in the Indian sub-population. Knowledge about normal variations in pigmentation is a prerequisite to discern abnormal pigmentary dermatoses.
| 1. |
Jablonski NG, Chaplin G. The evolution of human skin colouration. J Hum Evol 2000;39:57-106.
[Google Scholar]
|
| 2. |
Selmanowitz VJ, Krivo JM. Pigmentary demarcation lines. Comparison of Negroes with Japanese. Br J Dermatol 1975;93:371-7.
[Google Scholar]
|
| 3. |
McLaurin CI. Cutaneous reaction patterns in blacks. Dermatol Clin 1988;6:353-62.
[Google Scholar]
|
| 4. |
Henderson AL. Skin variations in blacks. Cutis 1983;32:376-7.
[Google Scholar]
|
| 5. |
Weary PE, Behlen CH 2nd. Unusual familial hypopigmentary anomaly. Arch Dermatol 1965;92:54-5.
[Google Scholar]
|
| 6. |
Selmanowitz VJ, Krivo JM. Hypopigmented markings in Negroes. Int J Dermatol 1973;12:229-35.
[Google Scholar]
|
| 7. |
Vazquez M, Ibanez MI, Sanchez JL. Pigmentary demarcation lines during pregnancy. Cutis 1986;38:263-6.
[Google Scholar]
|
| 8. |
James WD, Carter JM, Rodman OG. Pigmentary demarcation lines a population survey. J Am Acad Dermatol 1987;16:584-90.
[Google Scholar]
|
| 9. |
O'Brien TJ. The brown forehead ring: A pattern of facial pigmentation. Australas J Dermatol 1995;36:87-9.
[Google Scholar]
|
| 10. |
Futcher PH. A peculiarity of pigmentation of the upper arm of Negroes. Science 1938;88:570-1. Quoted by Victor J Selmanowitz and James M. Krivo. Br J Dermatol 1975;93:371.
[Google Scholar]
|
| 11. |
Miura O. A supplement to the demarcation lines of pigmentation observed among the Japanese. The Tohoku J Exp Med 1952;56:1.
[Google Scholar]
|
| 12. |
Vollum DI. Skin markings in Negro children from the West Indies. Br J Dermatol 1972;86:260-3.
[Google Scholar]
|
| 13. |
Ito K. The peculiar demarcation of pigmentation along the so-called Voigt's lines among the Japanese. Dermatologia Internationalis 1965;4:45.
[Google Scholar]
|
| 14. |
Miura O. On the demarcation lines of pigmentation observed among the Japanese. The Tohoku J Exp Med 1951;54:135.
[Google Scholar]
|
| 15. |
Malakar S, Dhar S. Pigmentary demarcation lines over the face. Dermatology 2000;200:85-6.
[Google Scholar]
|
| 16. |
Happle R. Dohi Memorial Lecture. New aspects of cutaneous mosaicism. J Dermatol 2002;29:681-92.
[Google Scholar]
|
| 17. |
Happle R. Mosaicism in human skin. Understanding the pattern and mechanisms. Arch Dermatol 1993;129:1460-70.
[Google Scholar]
|
| 18. |
Happle R. Pigmentary patterns associated with human mosaicism: A proposed classification. Eur J Dermatol 1993;3:170-4.
[Google Scholar]
|
| 19. |
Nordlund JJ, Ortonne JP. The normal colour of human skin. In: The Pigmentary System. Nordlund JJ, Biossy RE, Hearing VJ, King RA, Ortonne J-P, editors. Oxford: Oxford University Press; 1998. p. 475-87.
[Google Scholar]
|
Fulltext Views
39,251
PDF downloads
2,781





