Translate this page into:
Management of premenstrual acne with Cox-2 inhibitors: A placebo controlled study
2 Dept. of Endocrinology, M. S. Ramaiah Medical College, Bangalore - 560 054, India
Correspondence Address:
Rustom Tehrani
�Citadel�, 11 Plain Street, Shivajinagar, Bangalore - 560001
India
| How to cite this article: Tehrani R, Dharmalingam M. Management of premenstrual acne with Cox-2 inhibitors: A placebo controlled study. Indian J Dermatol Venereol Leprol 2004;70:345-348 |
Abstract
BACKGROUND: Premenstrual acne is poorly understood, the accepted hypothesis is 30 years old. AIMS: Here we test the hypothesis that premenstrual acne can be suppressed using Cox-2 inhibitors. METHODS: Eighty women with premenstrual acne were enrolled in a trial where they were given rofecoxib, a Cox-2 inhibitor or placebo for 10 days for two cycles and were evaluated using acne severity index and inflammatory acne counts. RESULTS: Rofecoxib was more effective than the placebo. CONCLUSIONS: Although the number studied is small, the results suggest that rofecoxib is effective in the management of premenstrual acne and that prostaglandin PGE2 may be involved in its pathogenesis.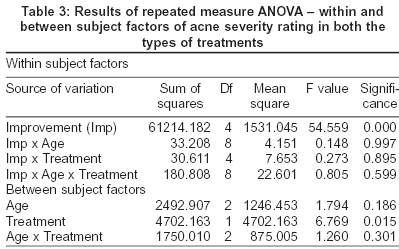



 |
 |
 |
 |
 |
 |
INTRODUCTION
Acne is one of the commonest maladies occurring in an individual′s lifetime. While acne responds to conventional therapy, premenstrual acne keeps recurring prior to menstruation and is refractory to treatment. Premenstrual acne occurs in 44% of women in the late luteal phase of menstruation.[1] A recent study showed that the incidence of premenstrual acne per menstrual period in these individuals was 63%.[2]
The accepted hypothesis, which is 30 years old, states that the pilosebaceous duct becomes smaller between days 15 and 20 of the menstrual cycle and the blockage leads to premenstrual acne.[3] However, the mechanism for this blockage is not known.
We propose that prostaglandins may be the causative agent for premenstrual acne through their vasoactive properties. Hence, we compared the effects of cyclical administration of a cyclooxygenase-2 (Cox-2) inhibitor, rofecoxib, with that of a placebo in patients with premenstrual acne.
METHODS
The study was undertaken in the year 2003-2004 in the dermatology outpatient department of a tertiary care hospital in Karnataka. After obtaining an informed consent, 80 consecutive women, with regular menstrual cycles and premenstrual acne were recruited in the study. The selection criteria were mild to moderate severity acne that had not been treated with corticosteroids or NSAIDs for one month prior to the trial.
Patients were randomly divided into two groups of 40 patients each, one group was put on rofecoxib 50 mg daily, while a control group was assigned to take a placebo, both starting 10 days prior to the onset of the menstrual cycle, or at the onset of premenstrual acne, if this occurred earlier and discontinued at the onset of menstruation.
Patients were studied for 2 cycles over 2 months. Patients were examined initially on enrollment, on Day 1 and Day 10 of the treatment by the severity index described by Michaelsson et al[4] by counting the number of open or closed comedones, papules, pustules and infiltrated lesions. They described the severity index as 0.5 for comedones, 1 for a papule, 2 for a pustule, 3 for infiltrated lesion and 4 for cystic lesions. Multiplying each type of lesion with its severity index and adding them together calculated the total severity score. An inflammatory acne count was done along with the severity rating. All patients were also advised to use a soothing cream (Aloederm©) daily.
RESULTS
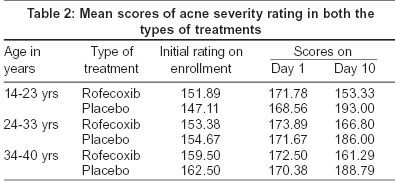
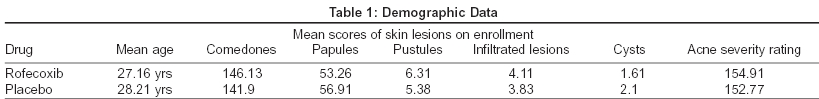
Eighty patients aged 14 to 45 years (mean age 27.65 years) were enrolled in the study. There were no significant differences in terms of demographic profile or acne severity between the two treatment groups [Table - 1]. Eleven patients failed to complete the study; six in the placebo group and five in the rofecoxib group. Reasons for premature withdrawal included lost to follow-up (placebo 3, rofecoxib 3), condition unchanged/worsened (placebo3, rofecoxib1) and 1 in the rofecoxib group because of severe epigastric pain. Efficacy results were based on 69 evaluable subjects (placebo 34, rofecoxib 35). These patients were divided into 3 groups age-wise and then examined statistically for acne severity rating and inflammatory acne counts.
[Table - 2] presents mean acne severity rating on enrollment, days 1 and 10. [Table - 3] shows the results of repeated measure ANOVA - within and between subject factors of acne severity rating in both the groups. We find from [Table - 2] that rofecoxib has significantly reduced the acne severity rating in all the three age groups in comparison to the placebo. In fact, the average acne severity rating in the placebo group worsened during the treatment period. From [Table - 3] it is clear that there is a definite improvement in the scores of acne severity rating and that this was statistically highly significant (F = 54.559, highly significant P< 0.0001).
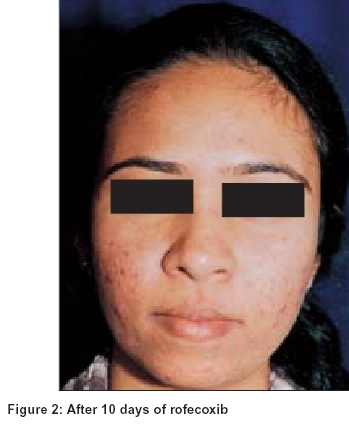
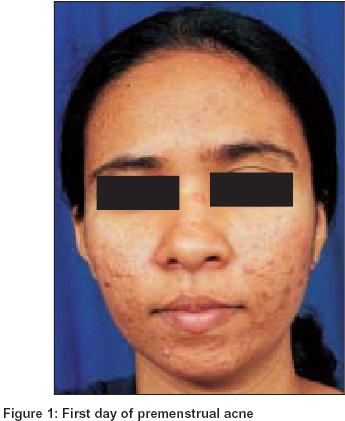
In evaluating the patients who responded to treatment it was observed that in the patients who took rofecoxib before the acne flare, there was usually no exaggeration of acne while those who took the drug at the onset of the acne flare, the acne generally subsided before their inter-menstrual period [Figure - 1], [Figure - 2].
Side-effects in the placebo group included malaise in one patient, while in the rofecoxib group one patient had severe epigastric discomfort, two had mild pedal edema and one developed photosensitivity. In the patient with epigastric discomfort the treatment had to be stopped whereas in the other cases it was not necessary to terminate therapy. In the placebo group one patient developed malaise and another headache; neither of these patients discontinued the drug.
DISCUSSION
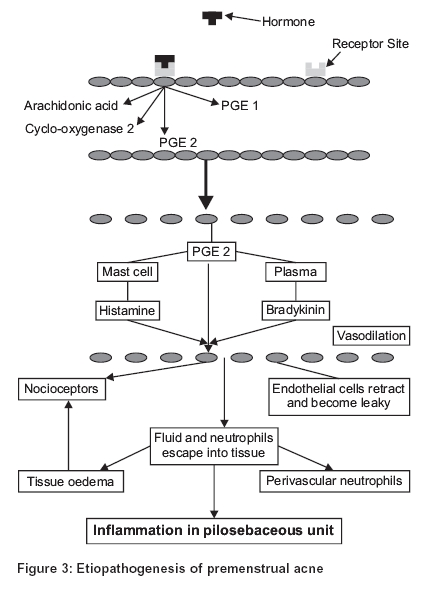
The traditional pathogenetic hypothesis of premenstrual acne based on passive pressure buildup has two major flaws; premenstrual acne is inflammatory in nature, and the fact that it is reversible when treated early, goes in favor of a biochemical pathogenesis rather than a pure obstructive pathology. The proposed etiopathogenesis of premenstrual acne is as follows [Figure - 3].
The triggering factor seems to be the female hormones, either estrogen or progesterone that bind to the hormone receptor sites on the cell membrane. This leads to the formation of prostaglandins of the PGE group from arachidonic acid. PGE2 causes the release of histamine from mast cells and the formation of bradykinin. This results in acute inflammation in the pilosebaceous unit causing premenstrual acne.[5]
Prostaglandins (PGE) are the most potent vasoactive substances produced in the body by the vascular endothelium from arachidonic acid by the action of the enzyme cyclo-oxygenase. They are not stored in the body but produced locally at the site of inflammation. The Cox-2 inhibitors act selectively by inhibiting the enzyme cyclooxygenase 2 that converts arachidonic acid to PGE2. It has no action on PGE1 synthesis, and hence the gastrointestinal side-effects are minimal.[6] A recent study from Israel concludes that premenstrual dermatological conditions are due to hypersensitivity to estrogens and progesterone.[7]
Corticosteroids have been used in different doses and duration, one treatment regime advocates the use of prednisolone 5 mg starting 10 days prior to the periods and discontinued at the onset of menstruation, with nearly 100% success and minimal side-effects.[8] This subjects a woman to corticosteroids for 30% of her reproductive life. The other corticosteroid regimes use daily doses of low-dose steroids to control acne in normal and hyperandrogenic states with improvement but no reference is made to its effect on premenstrual acne.
Contrary to popular belief oral contraceptives do not suppress premenstrual acne.[1] Rofecoxib selectively inhibits the Cox-2 enzyme and has minimal gastric irritation. It is FDA approved for osteo-arthritis, rheumatoid arthritis, dysmenorrhea and painful conditions.[9] However, prolonged administration of rofecoxib at a dose of 50 mg but not at a dose of 25 mg has been associated with an increased risk of myocardial ischemia.[10]
Both ibuprofen[11] and benoxaprofen[12] have been used successfully to treat acne. NSAIDs have also been used in the past to treat psoriasis, sunburn, erythema nodosum, cryoglobulinemia, Sweet′s syndrome, systemic mastocytosis as well as urticarial, livedoid and nodular vasculitis and their action is by blocking cyclooxygenase. The new exciting possibility is that non-melanotic cancers are mediated by PGE2; NSAIDs, especially COX-2 inhibitors are in the forefront of carcinoma research.[13]
The main limitations of traditional NSAID therapy are gastric intolerance and multiple daily dose schedules. If rofecoxib would be effective in a lower dosage it would be an ideal drug because of its once daily dosage and minimal side effects. It could usher a new era of selective NSAIDs treatment. This small study should be a predecessor to larger trials using selective Cox-2 inhibitors in a variety of inflammatory skin conditions including premenstrual acne along with biochemical studies. This study shows that Cox-2 inhibitors are useful in premenstrual acne not only prophylactically but also cause involution of early premenstrual acne.
ACKNOWLEDGMENTS
I am thankful to Dr. Gururaj Urs for the statistical analysis and Ms Shanaz Tehrani for the graphic portrayal of the etiopathogenesis of premenstrual acne.
| 1. |
Stoll S, Shalita AR, Webster GF, Kaplan R, Danesh S, Penstein A. The effect of the menstrual cycle on acne. J Am Acad Dermatol 2001;45:957-60.
[Google Scholar]
|
| 2. |
Lucky AW. Quantitative documentation of a premenstrual flare of facial acne in adult women. Arch Dermatol 2004;140:423-4.
[Google Scholar]
|
| 3. |
Williams M, Cunliffe WJ. Explanation for premenstrual acne. Lancet 1973;2:1055-7.
[Google Scholar]
|
| 4. |
Michaelsson G, Juhlin L, Vahlquist A. Effect of oral zinc and Vitamin A in acne. Arch Dermatol 1977;113:31-6.
[Google Scholar]
|
| 5. |
Tilley SL, Coffman TM, Koller BW. Mixed messages; modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest 2001;108:15-23.
[Google Scholar]
|
| 6. |
Smith KJ, Skeleton H. Arachidonic acid-derived bioactive Lipids: Their role and the role for their inhibitors in dermatology. J Cutan Med Surg 2002;6:241-56.
[Google Scholar]
|
| 7. |
Itesekson A, Lazarov A, Cordoba M, Zeitune M, Abraham D, Seidman DS. Premenstrual Syndrome and associated skin diseases related to hypersensitivity to female sex hormones. J Reprod Med 2004;49:195-9.
[Google Scholar]
|
| 8. |
Fischer DA. Desideratum Dermatologicum-cause and control of premenstrual acne flare. Int J Dermatol 2000;39:334-6.
[Google Scholar]
|
| 9. |
Katz N. Coxibs: Evolving role in pain management. Semin Arthritis Rheum 2002;32:15-24.
[Google Scholar]
|
| 10. |
Mukherjee D. Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001;286:954-9.
[Google Scholar]
|
| 11. |
Wong RC, Kang S, Heezen JL, Voorhees JJ, Ellis CN. Oral ibuprofen and tetracycline for the treatment of acne. J Am Acad Dermatol 1984;11:1076-81.
[Google Scholar]
|
| 12. |
Hindson C, Lawlor F, Wacks H. Benoxaprofen for nodular acne. Lancet 1982;1:1415.
[Google Scholar]
|
| 13. |
Friedman ES, LaNatra N, Stiller MJ. NSAIDs in dermatologic therapy: Review and preview. J Cutan Med Surg 2002;6:449-59.
[Google Scholar]
|
Fulltext Views
3,462
PDF downloads
1,226





