Translate this page into:
Tzanck smear: A useful diagnostic tool
Correspondence Address:
M K Singhi
H. NO. 3. MDM Doctors Qrtrs, MDM Hospital Road, Jodhpur, Rajasthan
India
| How to cite this article: Gupta LK, Singhi M K. Tzanck smear: A useful diagnostic tool. Indian J Dermatol Venereol Leprol 2005;71:295-299 |

 |
 |
 |
 |
 |
 |
 |
 |
Diagnostic cytology is a relatively new science. Cytology (Greek word: kytos = hollow vessel) is the study of individual cells and their intrinsic characteristics and functions. George Papanicolaou is considered the father of exfoliative cytology, but cytology was first used in cutaneous disorders by Tzanck in 1947, for the diagnosis of vesiculo-bullous disorders, particularly herpes simplex.[1] Since then cytology has been widely used by dermatologists for diagnosing various cutaneous dermatoses. [2],[3],[4],[5],[6],[7]
Cytodiagnosis is very simple, rapid, cheap and reliable. Its various methods are aspiration cytology, imprint smear, exudate smear, skin scraping smear, and Tzanck smear. Some practical applications of Tzanck smear in dermatological practice are summarized in [Table - 1]. Cytological diagnosis of tumors is not commonly used because surgical excision and biopsy are easy to perform. In some diseases, cytological findings are diagnostic, while in others they are only suggestive of a disease and need to be confirmed by histology.
Preparation of Tzanck smear
Tzanck smear is a very simple and rapid technique. For viral infections, samples should be taken from a fresh vesicle, rather than a crusted one, to ensure the yield of a number of virus infected cells. The vesicle should be unroofed or the crust removed, and the base scraped with a scalpel or the edge of a spatula. The material is transferred to a glass slide by touching the spatula to the glass slide repeatedly but gently. The slide should be clean, since cells will not adhere to a slide marred by fingerprints.
In the case of blistering disorders, the intact roof of a blister is opened along one side, folded back and the floor gently scraped. The material thus obtained is smeared onto a microscopic slide, allowed to air dry, and stained with Giemsa stain. If Papanicolaou stain is to be used, the slide should be immediately fixed in alcohol. Smearing bulla fluid and inclusion of blood may lead to inappropriate results.
For the cytodiagnosis of suspected tumors, any crust should be removed from ulcerated tumors, and non-ulcerated tumors should be incised with a sharp, pointed scalpel (the incision should be superficial enough to avoid undue bleeding). A sample of tumor is then obtained with either a blunt scalpel or a small curette, and the tissue obtained is pressed between the two slides.[8]
Fixation of Smear
A fixative is a fluid, often a mixture of several reactive chemicals, into which histological or cytological specimens are placed. Fixation is the use of a fixative to preserve histological or cytological specimens. It is needed to prevent denaturation and cross-linking of proteins, and autolysis, and to ensure that the specimen is hardened to withstand further processing and that both cellular morphology and the location of subcellular constituents are preserved in a close facsimile of the living state. A wide variety of fixatives, containing ingredients such as formalin, glutaraldehyde, other aldehydes, methanol, ethanol, other alcohols, acetone, acetic acid, chromates, and picric acid, are used for special purposes. Alcohol fixation can produce distortions and shrinkage unless used at a low temperature. An excellent cytological and tissue fixative is formol-Zenker solution, which consists of 9 parts Zenker stock solution and 1 part neutral formalin. The smear should preferably be fixed immediately since significant artifacts can result from drying.
Staining of Tzanck smear
Tzanck smear can be stained by a variety of methods, most commonly by Giemsa stain. Quick staining can be done by Hemacolorγ or Diff-Quikγ within 1 minute. Other stains used are hematoxylin and eosin, Wright, methylene blue, Papanicolaou and toluidine blue.
Giemsa stain
It is a solution containing azure II-eosin, glycerin and methanol. The stain is composed of methylene blue eosinate, azure A eosinate, azure B eosinate, and methylene blue chloride.
Method: The commercially available Giemsa stain solution is diluted 1:10 with distilled water, and the diluted solution is poured over the smear and kept for 15 minutes. Then it is washed with water and examined under the microscope. The stained nuclei may vary in color from reddish blue to purple to pink. The cytoplasm stains bluish.
A. CYTODIAGNOSIS OF IMMUNOBULLOUS DISORDERS
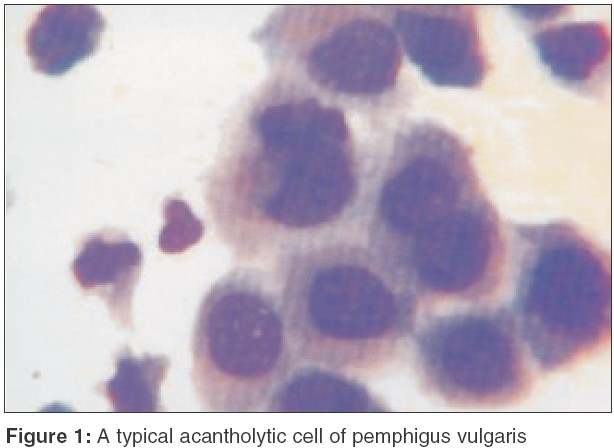
i) Pemphigus vulgaris:[2],[3],[4],[5] Tzanck test is very useful for the diagnosis of PV, particularly in the early stages of oral pemphigus where a biopsy is uncomfortable to the patient and of little help in clinching the diagnosis. It reveals multiple acantholytic cells (Tzanck cells). A typical Tzanck cell [Figure - 1] is a large round keratinocyte with a hypertrophic nucleus, hazy or absent nucleoli, and abundant basophilic cytoplasm. The basophilic staining is deeper peripherally on the cell membrane ("mourning edged" cells) due to the cytoplasm′s tendency to get condensed at the periphery, leading to a perinuclear halo.
Findings of cell adherence are relatively less characteristic cytological signs in pemphigus vulgaris.[3] A "Sertoli rosette" consists of cell aggregates with an epithelial cell at the center surrounded by a ring of leucocytes. "Streptocytes" are adherent chains of leukocytes formed by filamentous, glue like substances.
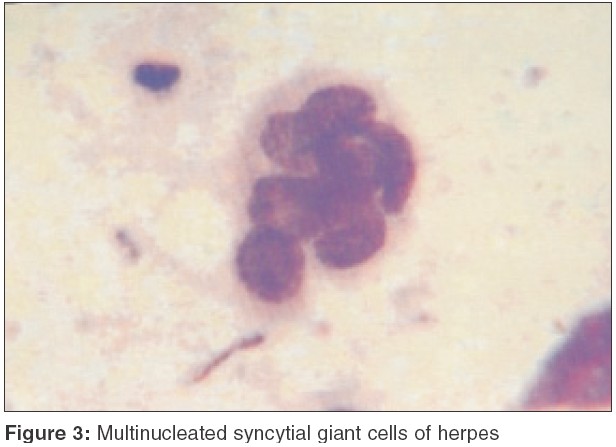
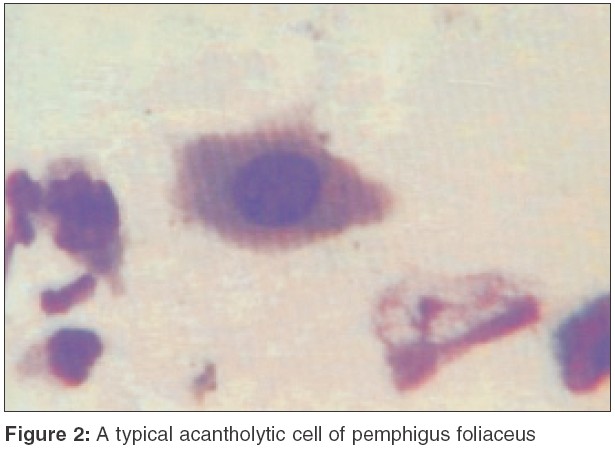
In pemphigus vegetans, the cytologic features are identical but there are usually more inflammatory cells, particularly eosinophils.[2] In contrast to pemphigus vulgaris, the acantholytic cells in pemphigus foliaceus and pemphigus erythematosus often have a hyalinized cytoplasm that corresponds to the dyskeratosis seen in tissue sections [Figure - 2].
ii) Toxic epidermal necrolysis (TEN) and staphylococcal scalded skin syndrome (SSSS)[2],[3]
It is extremely important to distinguish between these two conditions because of their differing treatment and prognosis. TEN is much more common in adults and SSSS in children. Although they can usually be easily distinguished clinically, there may be some overlap. Cytological examination may be helpful in such cases.[2] Smears from TEN show necrotizing or degenerating basal cells with scattered inflammatory cells and fibroblasts while those from SSSS show dyskeratotic acantholytic cells with very few inflammatory cells.[3] Cytodiagnosis should be substantiated either by a frozen section taken from the bulla roof or by a biopsy.
In bullous impetigo, dyskeratotic acantholytic cells may also be seen in large numbers, but they are usually associated with abundant neutrophils. Gram stained preparation may also show clusters of coccoid bacteria, which are not seen in SSSS (since the lesion is caused by a toxin and the bacteria may reside at a distant site).
iii) Bullous pemphigoid (BP), Stevens-Johnson syndrome (SJS) and erosive lichen planus:[2],[3] In these conditions, the findings of a Tzanck smear are non-specific and there are no acantholytic cells. The smear only serves to readily rule out pemphigus. Bullous pemphigoid shows scarcity of epithelial cells and an abundance of leukocytes, particularly eosinophils with leukocyte adherence. SJS and lichen planus may show altered or necrotic keratinocytes, leukocytes, fibrin filaments and rare fibroblasts.
B. CYTODIAGNOSIS OF CUTANEOUS INFECTIONS
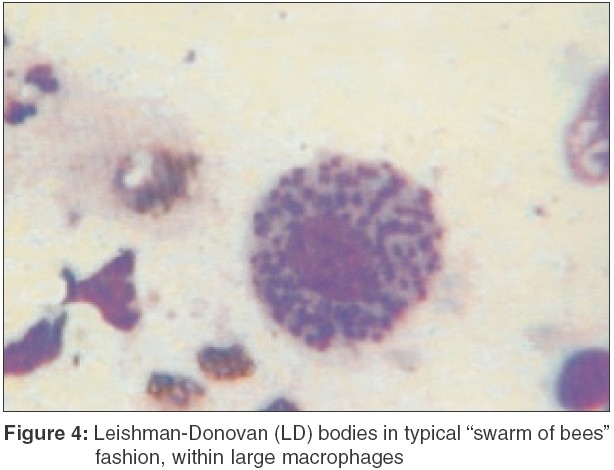
i) Herpes simplex, varicella, herpes zoster:[2],[3],[6] Infection by the herpes group of virus can be rapidly and reliably diagnosed by a Tzanck test. It may, however, be impossible to distinguish between these conditions based on cytodiagnostic features. Ideally, a vesicle less than 3 days old should be obtained since older lesions may get crusted or secondarily infected and the characteristic cytomorphology may no longer be present. The typical features include characteristic multinucleated syncytial giant cells and acantholytic cells [Figure - 3]. The cells appear as if they have been inflated ("ballooning degeneration") and sometimes may grow tremendously, 60-80 m in diameter. The giant cells often have a tadpole, bipolar, or irregular teardrop shape with a smooth external contour, in sharp contrast to the jagged configuration of sheets of abraded normal squamous epithelium. Syncytial giant cells contain multiple nuclei (many with 8 or more) that exhibit nuclear molding, so that the nuclei fit together in a jigsaw puzzle like fashion. The nuclei show great variation in shape and size. Intranuclear inclusion bodies surrounded by subtle clear halo are characteristic of herpetic infection, but are often difficult to find.
ii) Molluscum contagiosum:[2],[3] Although the clinical diagnosis is quite obvious, an isolated non-umbilicated lesion may be misdiagnosed for milium. Tzanck smear reveals the presence of diagnostic intracytoplasmic molluscum bodies (Henderson-Patterson bodies), the largest known inclusion bodies (30-35 m). They are virus-transformed keratinocytes, appearing as ovoid, deeply basophilic bodies with a hyaline, homogeneous structure surrounded by a membrane.
iii) Vaccinia, orf, milker′s nodules and variola:[2],[3] Despite a different clinical appearance, all these conditions exhibit similar cytological features. Smears show a variable number of acantholytic, or at least detached, squamous keratinocytes. They may contain an eosinophilic cytoplasmic inclusion called a "Guarnieri body", frequently surrounded by a clear halo. In orf and milker′s nodules, there is also a very prominent background of inflammatory cells and necrotic squamous keratinocytes.
iv) Pustular or bullous superficial fungal infections:[2],[3] Candida and geophilic or zoophilic strains of dermatophytes can present with pustules or large bullae. Although a potassium hydroxide preparation is quite helpful in diagnosis, the presence of hyphae or pseudohyphae can also be easily identified in Giemsa stained smears.
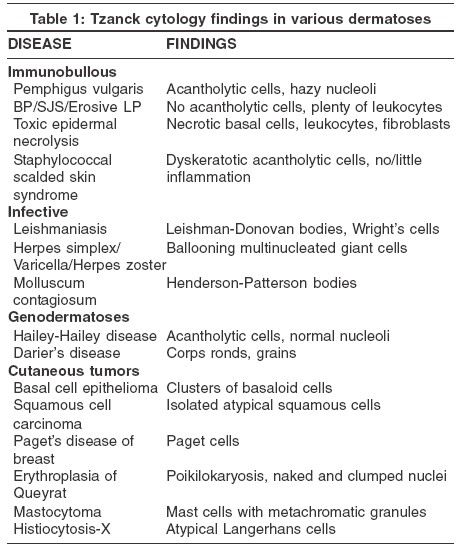
v) Leishmaniasis:[3] Cytology is very useful in detecting Leishman-Donovan (LD) bodies in early, untreated patients of leishmaniasis. LD bodies appear as light-blue, ellipsoid bodies, 2-4 m long, with an eccentric nucleus and a smaller kinetosome at the opposite pole. Numerous (20-30) protozoa are usually grouped together in a typical "swarm of bees" fashion [Figure - 4], within large macrophages (Wright′s cells). Isolated extracellular parasites are also found. Cytology may not be useful in chronic forms of leishmaniasis.
C. CYTODIAGNOSIS OF GENODERMATOSIS[3]
i) Hailey-Hailey disease: Cytodiagnosis can easily differentiate Hailey-Hailey disease from intertrigo, flexural psoriasis or eczema, which can closely mimic this genodermatosis. A Tzanck smear shows multiple acantholytic cells.
ii) Darier disease: Cytology in Darier disease reveals "corps ronds" and "grains." "Corps ronds" are isolated keratinocytes with a round shape and an acidophilic cytoplasm, which is retracted from the nucleus and denser peripherally ("mantle cells"). The grains are seen as small, hyaline, acidophilic ovoid bodies resembling pomegranate seeds.
iii) Vesicular and pustular dermatosis in neonates : Smears of pustules in transient neonatal pustulosis and infantile acropustulosis show predominance of neutrophils. Smears of pustules in eosinophilic pustulosis show plentiful eosinophils.
D. CYTODIAGNOSIS OF CUTANEOUS TUMORS
i) Basal cell epithelioma:[2],[3],[7] A Tzanck smear offers a high degree of reliability in the diagnosis of basal cell epithelioma. It shows clusters of basaloid cells, some of which may exhibit retention of peripheral palisading, as in the histology. Basaloid cells look like normal basal cells except for being a little larger and more deeply basophilic. They are uniform sized, elongated and have a central oval, intensely basophilic nucleus which occupies four-fifths of the cells. The cytoplasm is scant, poorly defined and basophilic, and may contain melanin granules, particularly in the pigmented variant of the tumor.
ii) Squamous cell carcinoma:[2],[3] Cytology is helpful in the nodular, soft or ulcerated non-keratotic (oral/genital locations) varieties of squamous cell carcinoma, but not in keratotic or verrucous lesions. The two distinctive cytological features of squamous cell carcinoma are the tendency of cells to be isolated (absence of clusters), and pleomorphism. Higher magnification shows nuclear alterations (hypertrophic, hyperchromatic, lobated or multiple nuclei, and abnormal mitoses) and bizarre changes in cytoplasm staining (basophilic in some, acidophilic in others).
iii) Paget′s disease:[3] Paget′s cells can be easily visualized on Tzanck cytology. They occur singly or in small groups, and are round to oval cells with amphophilic, vacuolated cytoplasm and a hypertrophic nucleolated nucleus. They appear larger than keratinocytes. Special stains for epithelial mucin (mucicarmine periodic acid-Schiff stain) can further corroborate the diagnosis by staining most Paget′s cells.
iv) Erythroplasia of Queyrat:[3] A smear shows polyhedral, spindle-shaped and round cells with "poikilokaryosis" (nuclear polymorphism relating to size, shape and staining), which is practically diagnostic for this intraepithelial carcinoma.
v) Mastocytoma:[3] Cytodiagnosis of mastocytoma is especially useful in children, in whom the need for biopsy may be obviated. Tzanck smear stained by 1% methylene solution for 1 minute shows plenty of mast cells, which are recognized by their irregular shape (triangular, polygonal, or pyriform) and metachromatic staining of granules (reddish purple).
vi) Histiocytosis X:[3] Multinucleate atypical Langerhans cells appear as 12-15 mm sized cells with wide, pale, weakly eosinophilic or amphophilic, micro-vacuolated or granular cytoplasm and a large lobulated, convoluted, reniform or centrally grooved nucleus. The cytodiagnostic findings, although quite suggestive, should always be confirmed by histology and immunophenotyping.
CONCLUSIONS
Despite the exponential growth and interest in dermatopathology over the years and the fact that the skin is the largest desquamating organ in the body, interest in cutaneous cytology has been limited. Although not a substitute for standard histology, in the hands of an experienced dermatologist Tzanck smears can aid in establishing the clinical diagnosis with ease and rapidity and can serve as an adjunct to routine histologic study. The technique is cheap, easy to perform and does not cause any discomfort to the patient.
| 1. |
Tzanck A. Le cytodiagnostic immediate en dermatologie. Bull Soc Fr Dermatol Syph 1947;7:68 (Quoted from Barr RJ, Irvine CA. Cutaneous cytology. J Am Acad Dermatol 1984;10:163-80).
[Google Scholar]
|
| 2. |
Barr RJ. Cutaneous cytology. J Am Acad Dermatol 1984;10:163-80.
[Google Scholar]
|
| 3. |
Ruocco V, Ruocco E. Tzanck smear, an old test for the new millennium: when and how? Int J Dermatol 1999;38:830-4.
[Google Scholar]
|
| 4. |
Graham JH, Bingul O, Burgoon CF Jr. Cytodiagnosis of inflammatory dermatosis: Pitfalls in evaluation of smears. Arch Dermatol 1963;87:18-27.
[Google Scholar]
|
| 5. |
Blank H, Burgoon CF Jr. Abnormal cytology of epithelial cells in pemphigus vulgaris: A diagnostic aid. J Invest Dermatol 1952;18:213-23.
[Google Scholar]
|
| 6. |
Solomon AR, Rasmussen JE, Weiss JS. A comparison of the Tzanck smear and viral isolation in varicella and herpes zoster. Arch Dermatol 1986;122:282-5.
[Google Scholar]
|
| 7. |
Oram Y, Turhan O, Aydin NE. Diagnostic value of cytology in basal cell and squamous cell carcinomas. Int J Dermatol 1997;36:156-7.
[Google Scholar]
|
| 8. |
Cerio R, Calonje E. Histopathology of the skin. General principles. In : Burns T, Stephen B, Cox N, Griffiths C, editors. Rook's Textbook of dermatology. 7th edn. Oxford: Blackwell; 2004. p. 726-7.
[Google Scholar]
|
Fulltext Views
114,599
PDF downloads
11,275





