Translate this page into:
An evaluation of the efficacy and safety of tazarotene (0.1%) cream in acne vulgaris
Correspondence Address:
P K Nigam
Department of Dermatology-Venereology, D-30-A, Shailendra Nagar, Raipur-492 001
India
| How to cite this article: Nigam P K, Anant S. An evaluation of the efficacy and safety of tazarotene (0.1%) cream in acne vulgaris. Indian J Dermatol Venereol Leprol 2005;71:360-361 |

Sir,
Systemic retinoids have long been used in the treatment of acne. Topical retinoids were developed to avoid many of their side effects. Tazarotene is a receptor selective retinoid[1] with low systemic absorption, rapid elimination and plasma concentration about 100 times lower than those observed after oral therapy with isotretinoin. It normalizes keratinocyte differentiation, reverses keratinocyte hyper-proliferation, and has anti-inflammatory effects.[2] We evaluated the efficacy and safety of 0.1% tazarotene cream in acne vulgaris.
The study was conducted in 46 consecutive patients with mild to moderate acne, as classified by Bershad et al,[3] i.e. 10-200 non-inflammatory lesions (open and closed comedones), 10-60 inflammatory lesions (papules and pustules), and fewer than 3 nodulocystic lesions, after due consent. Patients with severe nodulocystic acne were not included in the study. None of the patients was on systemic retinoids prior to the study. A washout period of two weeks was required for topical acne medications. Female patients were excluded if they were pregnant, breastfeeding or sexually active and not using contraception. Patients with known hepatic or renal disease were also excluded from the study.
Tazarotene 0.1% cream (a uniform brand) was applied as a thin film over the affected area once daily in the evening after washing the area with soap and water. The response was evaluated at 2, 4, 8 and 12 weeks. Simultaneously, the patients were also evaluated for any local side effects, including skin erythema, burning, peeling and tenderness at baseline and throughout the study. Data was analyzed using the paired ′t′ test.
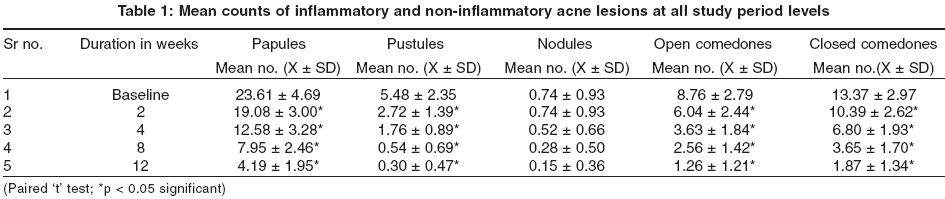
There were 20 males and 26 females, their age ranging from 17 years to 29 years (mean age, 22.48 ± 2.7 years). The mean count of different lesions at baseline and at 2, 4, 8 and 12 weeks are shown in [Table - 1]. A statistically significant reduction was seen at 8 and 12 weeks in the mean number of papules (66.3% and 82.3%), pustules (90.1% and 94.5%), nodules (62.2% and 79.7%), open comedones (70.8% and 85.6%), and closed comedones (72.7% and 86.1%) respectively. There was a statistically significant reduction in both the inflammatory and non-inflammatory lesion count at all study periods. The mean inflammatory acne count decreased by 70.6% and 84.5%, and the non-inflammatory acne count decreased by 71.9% and 85.8% at 8 weeks and 12 weeks respectively. The mean total lesion count reduced from 51.9 at baseline to 14.9 (28.8%) and 7.8 (14.9%) at 8 weeks and 12 weeks, which was also a significant reduction.
Adverse effects were noticed in 5 (10.8%) patients, in the form of mild burning in 3 (6.5%), itching in 3 (6.5%), erythema in 2 (4.3%) and desquamation in one (2.2%) patient. Three of these patients initially intolerant of the treatment decided to continue the treatment on reducing the application time to 15 minutes/day in the evening. Two patients discontinued the treatment and asked to be shifted to another drug. In one study with overnight topical tazarotene therapy, 9% of patients withdrew due to local irritation.[4] In another study, untoward effects were experienced by 11.9% of patients during the treatment.[5]
Topical tazarotene has been observed to show beneficial effects in acne vulgaris.[5]-[6] The efficacy and tolerability of tazarotene has been found equal to or superior to tretinoin 0.1% gel[3] and adapalene 0.1% gel.[6] Except for ovulating females, where due safety measures should be taken, topical tazarotene appears to be a safe and effective topical remedy for acne vulgaris.
| 1. |
Nagpal S, Patel S, Jacobe H, DiSepio D, Ghosn C, Malhorta M, et al. Tazarotene induced gene 2 (TIG 2), a novel retinoid responsive gene in skin. J Invest Dermatol 1997;109:91-5.
[Google Scholar]
|
| 2. |
Menter A. Pharmacokinetics and safety of tazarotene. J Am Acad Dermatol 2000;43:S31-5.
[Google Scholar]
|
| 3. |
Bershad S, Kranjac Singer G, Parente JE, Tan MH, Sherer DW, Persaud AN, et al. Successful treatment of acne vulgaris using a new method: Results of a randomized vehicle-controlled trial of short-contact therapy with 0.1% tazarotene gel. Arch Dermatol 2002;138:481-9.
[Google Scholar]
|
| 4. |
Shalita AR, Chalker DK, Griffith RF, Herbert AA, Hickman JG, Maloney JM, et al. Tazarotene gel is safe and effective in the treatment of acne vulgaris: a multicenter, double-blind, vehicle-controlled study. Cutis 1999;63:349-54.
[Google Scholar]
|
| 5. |
Saple DG, Torsekar RG, Pawanarkar V, Dhanalakshmi UR, Ravichandran G, Kaur D, et al. An open study to evaluate the efficacy and safety of tazarotene gel (0.1%) in acne vulgaris. Indian J Dermatol Venereol Leprol 2004;70:92-5.
[Google Scholar]
|
| 6. |
Webster GF, Guenther L, Poulin YP, Solomon BA, Lowen K, Lee J, et al. A multicenter double blind, randomized comparision study of the efficacy and tolerability of once daily tazarotene 0.1% gel and adapalene 0.1% gel for the treatment of facial acne. Cutis 2002;69:4-11.
[Google Scholar]
|
Fulltext Views
3,372
PDF downloads
1,130





