Translate this page into:
Granulomatous reaction following bacillus Calmette–Guérin vaccination: Successful response to clarithromycin
Corresponding author: Dr. Arti Nanda, P.O. Box: 6759, Salmiya 22078, Kuwait. artinanda@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Nanda A, Al-Sabah H, Al-Sumait A, AlNaqi N, Al-Otaibi M, AlLafi A. Granulomatous reaction following bacillus Calmette–Guérin vaccination: Successful response to clarithromycin. Indian J Dermatol Venereol Leprol 2021;87:816-8.
Abstract
We report a 3-year-old girl with a delayed nontuberculous granulomatous reaction on a bacillus Calmette–Guérin injection site with dissemination to distant sites who showed a favorable response to clarithromycin used for 12 weeks with no recurrence on a follow-up of more than 2 years.
Keywords
Bacillus Calmette–Guérin vaccination
clarithromycin
granuloma
Introduction
Bacillus Calmette–Guérin vaccine is an attenuated strain of Mycobacterium bovis (M. bovis) widely used as a preventive strategy against tuberculosis. Local adverse reactions at the site of bacillus Calmette–Guérin vaccination have been estimated to occur in 0.1 to 0.5 per 1000 vaccines.1 Disseminated bacillus Calmette–Guérin infection is rare and usually reported in patients with underlying immunodeficiency state.2 Among dermatological complications, both specific and nonspecific complications have been reported.3 The nonspecific complications include erythema, swelling, erosions or ulceration, abscess formation, hypertrophic scarring and keloids. Specific complications include lupus vulgaris, tuberculides, Koch phenomenon-like reaction, local subcutaneous abscess and scrofuloderma.3,4 Rarely, there have been reports of nontuberculous granulomatous reactions.5-8
Whereas the patients with tuberculous granulomatous reactions including lupus vulgaris and scrofuloderma are treated with antitubercular therapy, there is no consensus about the treatment of nontuberculous granulomatous reactions. We report a patient with delayed nontuberculous granulomatous reaction following bacillus Calmette–Guérin vaccination who was treated successfully with clarithromycin.
Case Report
A 3-year-old girl was referred to us with a 3 week history of a rapidly progressive asymptomatic scaly rash on the left arm at the site of bacillus Calmette–Guérin vaccination and three distant lesions on both the legs. She had received a bacillus Calmette–Guérin vaccination at the age of 3 months and was noticed to have a scaly papule at the vaccination site that never healed completely and subsequently extended slowly. The lesions had failed to respond to various topical modalities including antibiotics, mid-potency steroids and moisturizers. There were no associated medical complaints. Her birth history and developmental milestones were reported normal. A family history of tuberculosis was denied. On examination, she was afebrile. Her height and weight were below fifth percentile. She was observed to have large annular erythematous scaly plaque (9 × 10 cm) showing central clearing with mild atrophy and peripheral activity [Figure 1a] on the left arm, one small erythematous scaly plaque about 2 cm in diameter on the left leg [Figure 1b] and two small papules on the right leg. There was no associated lymphadenopathy or hepatosplenomegaly.
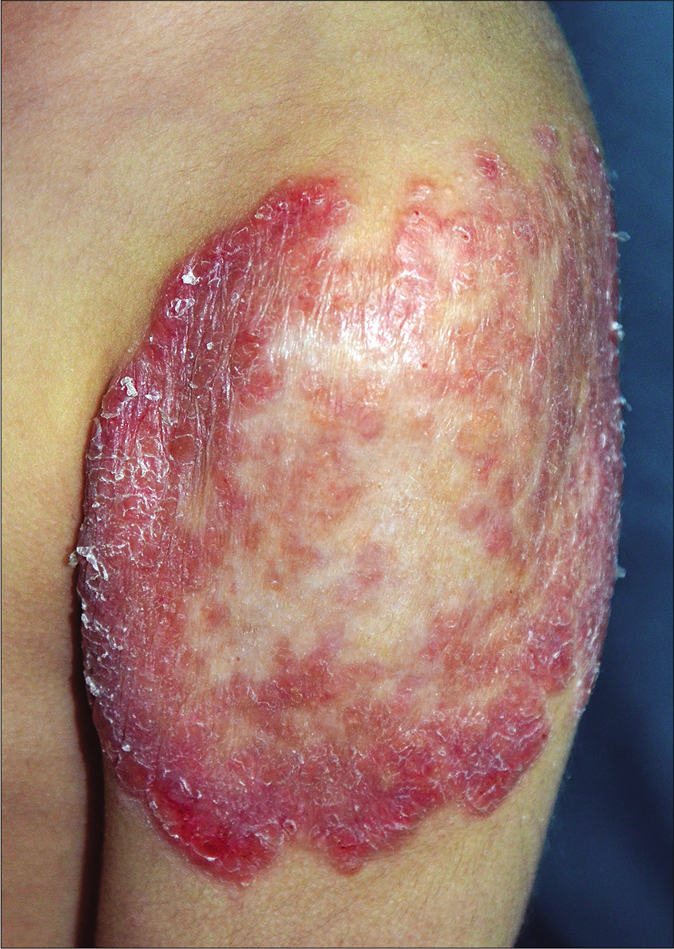
- Erythematous large plaque showing central clearing with mild atrophy, erosions in the periphery (lower border) and few satellite nodules near the upper border
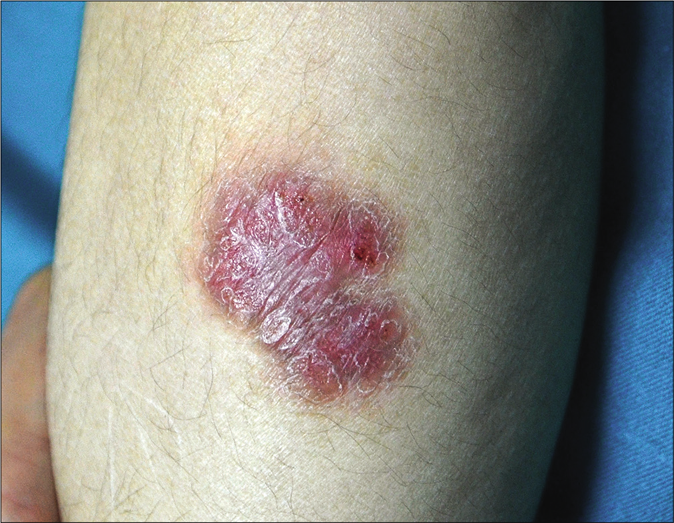
- Erythematous plaque on the left leg
Skin biopsies for histopathology from the lesion on the left arm and right leg showed similar histological features including marked acanthosis of the epidermis with multiple epithelioid cell granulomas with multiple foreign body multinucleated giant cells surrounded by dense lymphocytic infiltrate in both superficial and deep dermis [Figure 2a and b]. There was no evidence of caseation necrosis. Special stains including periodic acid-Schiff, periodic acid-Schiff-D and Ziehl-Neelsen were negative. Mycobacterial culture and polymerase chain reaction for Mycobacterium tuberculosis on skin biopsy yielded negative results. The Mycobacterium tuberculosis interferon gamma release assay (IGRA) and T-spot tuberculosis test were negative. Her erythrocyte sedimentation rate was 81 mm/h. Various other investigations including complete blood counts, serum biochemistry, liver and renal profile, serum immunoglobulins, T- and B-cell counts and immunophenotyping, chest X ray and ultrasound abdomen and pelvis were reported normal. An evaluation by a pediatric immunologist ruled out any possibility of underlying immunocompromised state including primary immunodeficiency. She was treated with clarithromycin 15 mg/kg/day in two divided doses for 12 weeks with complete clearance [Figure 3]. There was no recurrence on a follow up of two years.
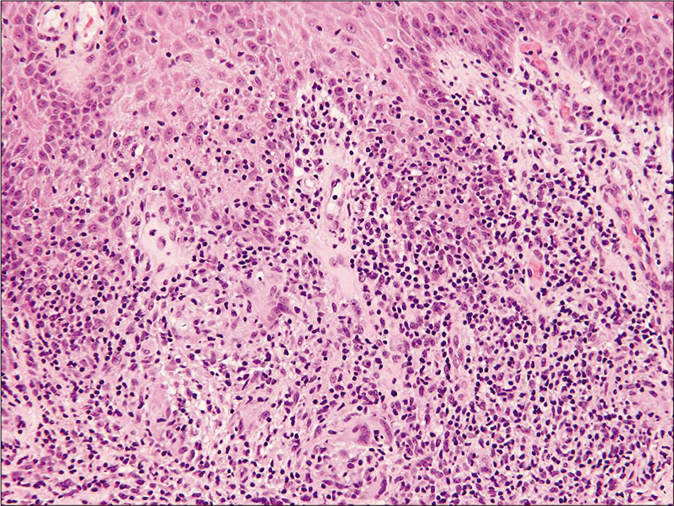
- Histopathology of skin biopsy from left-arm showing nodular granulomatous infiltrate of epithelioid cells and multinucleated giant cells surrounded by dense lymphocytic infiltrate (H and E, ×100)
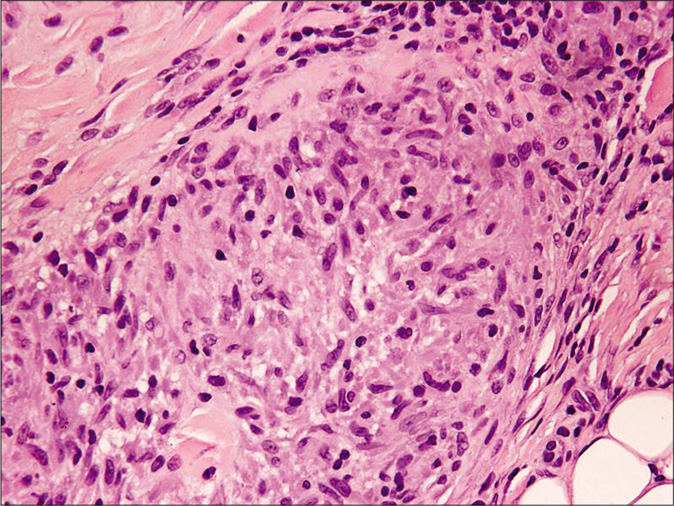
- Histopathology of skin biopsy from left-arm showing well-formed epithelioid cell granuloma in the deep dermis (H and E, ×400)
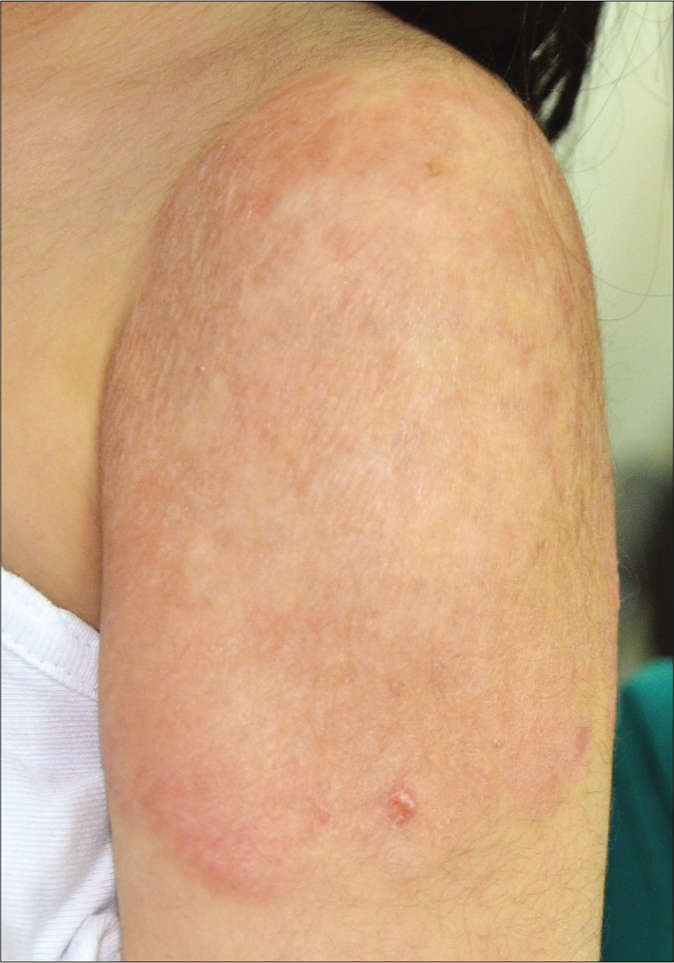
- Lesion showing complete healing with some atrophy and dyspigmentation after 3 months of clarithromycin therapy
Discussion
Nontuberculous granulomas at bacillus Calmette–Guérin site refer to granulomas that fail to show acid-fast bacilli by special stains, tissue culture or polymerase chain reaction and demonstrate negative relevant blood tests for tuberculosis including interferon-gamma release assay and T-spot tests. The exact pathogenesis of such granulomatous reactions following bacillus Calmette–Guérin vaccination is not certain. It has been proposed that granuloma formation in such patients can be related to a hypersensitivity reaction to the protein content in the vaccination.5,7,8 Chiu et al.6 demonstrated the presence of monosodium glutamate crystals (used as a stabilizer in the bacillus Calmette–Guérin vaccine) in the biopsy specimen from the granuloma and proposed them to be responsible for initiating a foreign body granulomatous reaction in their patient. However, a possibility of the presence of M. bovis strain of low virulence in undetectable amounts in the tissues to be responsible for granulomatous reaction cannot be fully ruled out. Whether there are some other factors that participate in granuloma formation in such cases need to be determined. Because all measures to identify acid-fast bacilli were negative in our patient, he was diagnosed to be having a nontuberculous granulomatous reaction. There is no consensus on treatment of such reactions. Various treatments including topical steroids, curettage and cautery, and antitubercular treatment have been used.5-8 We decided to use systemic therapy due to rapidly progressive large granulomatous lesion on bacillus Calmette– Guérin site, dissemination to distant sites and a prior history of failure to respond to topical steroids used by a primary physician. Clarithromycin monotherapy has previously been reported to be effective in a patient with bacillus Calmette– Guérin-induced regional complications.9 Before considering multidrug therapy with antitubercular treatment for a period of 6 months, we preferred to give a trial with clarithromycin monotherapy and our patient showed a remarkable response. Exact mechanism of action how clarithromycin helps in improving the granulomatous reaction is not clear. In addition to its antimycobacterial susceptibility against M. bovis,10 clarithromycin has also been documented to have antiinflammatory and immunomodulatory effect in patients with cystic fibrosis lung disease.11 Our patient was noticed to have an improvement within 2 weeks of clarithromycin treatment with a complete clearance by the end of 12 weeks.
To conclude, we report a patient with a delayed granulomatous reaction on bacillus Calmette–Guérin vaccination site with dissemination to distant sites who showed a favorable response to clarithromycin. We propose a trial of clarithromycin monotherapy in such patients prior to treatment with conventional multidrug antitubercular treatment is considered.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the legal guardian has given his consent for images and other clinical information to be reported in the journal. The guardian understands that names and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- BCG complications. Estimates of the risks among vaccinated subjects and statistical analysis of their main characteristics. Adv Tuberc Res. 1984;21:107-93.
- [Google Scholar]
- Dermatological complications of BCG vaccination. Br J Dermatol. 1963;75:181-92.
- [CrossRef] [Google Scholar]
- Disseminated Bacillus Calmette-Guérin infection and immunodeficiency. Emerg Infect Dis. 2007;13:799-801.
- [CrossRef] [PubMed] [Google Scholar]
- Disseminated cutaneous eruption after BCG vaccination. Pediatr Dermatol. 1996;13:451-4.
- [CrossRef] [PubMed] [Google Scholar]
- Delayed granulomatous lesion at the Bacillus Calmette-Guérin vaccination site. Acta Derm Venereol. 2001;81:302-4.
- [CrossRef] [PubMed] [Google Scholar]
- Foreign body granuloma caused by monosodium glutamate after BCG vaccination. J Am Acad Dermatol. 2006;55:S1-5.
- [CrossRef] [PubMed] [Google Scholar]
- A lesion at BCG vaccination site. Clin Exp Dermatol. 2009;34:117-8.
- [CrossRef] [PubMed] [Google Scholar]
- Granuloma annulare-like reaction to the Bacillus Calmette-Guerin vaccination. Australas J Dermatol. 2013;54:e4-7.
- [CrossRef] [PubMed] [Google Scholar]
- Short course of clarithromycin in an immunocompetent patient with BCG-induced regional complications. Dermatol Online J. 2002;8:6.
- [Google Scholar]
- Susceptibility pattern of Bacilli Calmette-Guerin strains against pyrazinamide and other major anti-mycobacterial drugs. Arch Pediatr Infect Dis. 2015;3:e17814.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and immunomodulating effects of clarithromycin in patients with cystic fibrosis lung disease. Mediators Inflamm. 2004;13:111-7.
- [CrossRef] [PubMed] [Google Scholar]






