Translate this page into:
Liposomal amphotericin B is more effective in polymorphic lesions of post kala-azar dermal leishmaniasis
Corresponding author: Prof. Mitali Chatterjee, Department of Pharmacology, Institute of Postgraduate Medical Education and Research, 244 B, Acharya J C Bose Road, Kolkata - 700 020, West Bengal, India. ilatimc@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Moulik S, Sengupta R, Ghosh MK, Das NK, Saha B, Chatterjee M. Liposomal amphotericin B is more effective in polymorphic lesions of post kala-azar dermal leishmaniasis. Indian J Dermatol Venereol Leprol 2022;88:201-6.
Abstract
Background:
Post kala-azar dermal leishmaniasis (PKDL) is thought to be the reservoir of infection for visceral leishmaniasis in South Asia. The development of strategies for the diagnosis and treatment of PKDL are important for the implementation of the visceral leishmaniasis elimination program.
Aims:
Liposomal amphotericin B (L-AMB) has been an overwhelming success in the treatment of visceral leishmaniasis. However, the empirical three-week regimen of L-AMB proposed for PKDL was shown to be inadequate, especially in the macular variant. This study aimed to delineate response of the different variants of PKDL to L-AMB.
Methods:
Skin biopsies were collected from PKDL cases at disease presentation and upon completion of treatment with L-AMB. Parasite DNA was detected by Internal Transcribed Spacer-1 PCR (ITS-1 PCR) and quantified by amplification of parasite kDNA. CD68 + macrophages were estimated in tissue sections by immunohistochemistry.
Results:
Treatment with L-AMB decreased the parasite load by 97% in polymorphic cases but only by 45% in macular cases. The median parasite load (89965 vs 5445 parasites/μg of genomic DNA) as well as infiltration by CD68+ cells before treatment was much greater in the polymorphic cases.
Limitations:
Although monitoring of the parasite load for 12 months post-treatment would have been ideal, this was not possible owing to logistical issues as well as the invasive nature of biopsy collection procedure.
Conclusion:
A dramatic decrease in the parasite burden was noted in patients with polymorphic lesions. Although patients with macular disease also had a decrease in parasite burden, this was not as marked as in the polymorphic cases. There was also a significantly greater infiltration of CD68 + macrophages in polymorphic PKDL before therapy.
Keywords
Liposomal amphotericin B
macrophages
macular post kala-azar dermal leishmaniasis
parasite load
polymorphic post kala-azar dermal leishmaniasis
Introduction
An estimated 200,000–400,000 new cases of kala-azar (visceral leishmaniasis) caused by Leishmania donovani occur worldwide annually with more than two third of these cases occurring in India, Bangladesh and Nepal.1
The Governments of these three countries, supported by the World Health Organization, jointly launched a visceral leishmaniasis elimination initiative in 2005 targeting a reduction in the annual incidence to below 1/10,000 people by 2015, a deadline later reset to 2020.2 This program is presently in the consolidation phase and includes active surveillance to detect the potential disease reservoirs (i.e., asymptomatic cases and patients with PKDL).
PKDL is one of the most challenging types of leishmaniasis, both in terms of its etiopathogenesis as well as management.3,4 Macular PKDL presents with hypopigmented or erythematous lesions, while the polymorphic variant manifests as a combination of macules, papules and/or nodules. Both variants harbor parasites that are easily accessible to the sandfly, making them key players in the transmission cycle.3,4
The active case detection strategy for leishmaniasis has unearthed a vast number of macular PKDL cases. These macular cases pose a diagnostic dilemma owing to the scanty presence of parasites in these lesions.5 The diagnosis of the hypopigmented PKDL lesions is usually based on clinical features that are often indistinguishable from other diseases such as vitiligo, pityriasis versicolor and leprosy.6,7 Patients with macular lesions are less likely to seek treatment as compared to those with disfiguring papular and nodular lesions. 5 Further, as the hypopigmentation persists even after the elimination of parasites, quantification of parasite load becomes a critical parameter of efficacy.
An empirical three-week regimen was proposed for PKDL but this proved inadequate in eliminating parasites especially in the macular variant.8,9 Polymorphic and macular PKDL have been demonstrated to have differences in immune responses and host-pathogen interactions.7 The present study aimed to delineate the differential response of patients with macular and polymorphic PKDL treated with L-AMB and establish a correlation, if any with the degree of macrophage infiltration.
Methods
Study population
Thirty-eight patients clinically diagnosed with PKDL were recruited by:
Passive surveillance from the dermatology outpatient departments of School of Tropical Medicine, Calcutta Medical College, IPGME&R, Kolkata, West Bengal, and
Active surveillance by field surveys conducted in endemic districts of West Bengal (Malda, Dakshin Dinajpur, Murshidabad and Birbhum).8
The Institutional Ethics Committees approved the study, and all patients or their legally accepted representative provided informed written consent. Blood samples and skin biopsies were collected both before and after treatment. The diagnosis of PKDL was made by the demonstration of rK39-ELISA positivity and confirmed by ITS-1 PCR in the biopsies. All cases received L-AMB (5 mg/kg body IV, twice weekly for three weeks).8
Diagnosis by ITS-1 PCR
DNA extraction was performed according to manufacturer’s instructions from a skin biopsy and PCR was performed using Leishmania-specific primers as previously described.8
Measurement of parasite load by real-time PCR
The parasite load was extrapolated from a standard curve generated by adding a defined number of Leishmania parasites and real-time PCR performed using specific primers for minicircle kinetoplast DNA.8 The parasite number when <10 reported a Ct value almost equivalent to non-template control, and was accorded an arbitrary value of one.
Immunohistochemistry
Formalin-fixed paraffin-embedded sections were incubated with EnVision™ FLEX Mini Kit, High pH (DAB+ chromogen) as per the manufacturer’s protocol; sections from skin of healthy individuals served as positive controls.10 For healthy controls, 4-mm skin biopsies were obtained from laboratory volunteers or the foreskin of males undergoing voluntary circumcision. The average of five manually counted fields at 400 × magnification was expressed as cells mm−2.
Statistical analysis
Data was expressed as median (inter quartile range). When three groups were compared, nonparametric data was analyzed between groups by the Kruskal–Wallis test followed by Dunn’s multiple comparison test. Wilcoxon signed rank test was done for paired samples using the Graph Pad Prism Software version 5.0 (GraphPad Software Inc., La Jolla, CA, USA);P < 0.05 was considered significant.
Results
Study population
The study included 38 patients diagnosed with PKDL. Twelve of these were sourced by passive surveillance (10 polymorphic, 2 macular) while 26 patients were detected by active surveillance (15 polymorphic, 11 macular). All patients were monitored until completion of treatment [Table 1].
| Clinical features | Patients with PKDL (n=38) | |
|---|---|---|
| Polymorphic (n=21) | Macular (n=17) | |
| Age (years) | 23 (18-35)* | 17 (10-26)* |
| Gender (male:female) | 2:1 | 1.42:1 |
| Interval between cure of visceral leishmaniasis and onset of PKDL (years) | 7 (5-11)* | 6 (3-7)* |
| Duration of PKDL/patient delay (years) | 3 (1-5)* | 3 (1-3)* |
Treatment led to a dramatic decrease in parasite load in polymorphic cases
All 38 patients were ITS-1 PCR positive both before and after completion of treatment. The median parasite load before treatment in polymorphic cases was over 16-fold higher than in the macular variant (89,965 vs 5445 parasites/μg of genomic DNA). After treatment there was a dramatic 97% decrease in the parasite load in polymorphic cases as compared to a modest 45% decrease in the macular cases [Figure 1a].
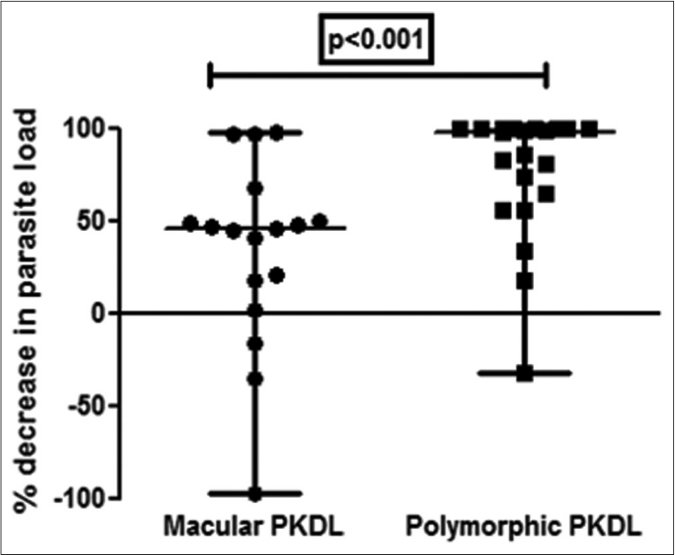
- Scatter plots showing the percent decrease in parasite load as median (interquartile range) in patients with polymorphic post kala-azar dermal leishmaniasis (●, n= 21) vs. macular post kala-azar dermal leishmaniasis (◼, n= 17) after completion of treatment with liposomal amphotericin b.
A >50% decrease in parasite load was seen in 18/21 (85.7%) of the polymorphic cases as compared to only 4/17 (23.5%) of the macular cases [Figures 1b-h]. This correlated with the considerable decrease in the lesions of the majority of the polymorphic cases following completion of treatment [Figure 1i]; however, the clinical features persisted in the macular cases [Figure 1j].
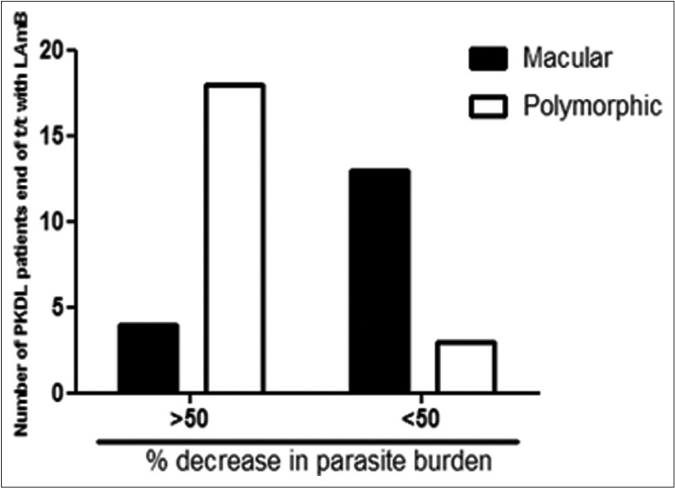
- Bar diagram indicating the number of patients with polymorphic or macular post kala-azar dermal leishmaniasis who demonstrated >50% and <50% decrease in parasite burden
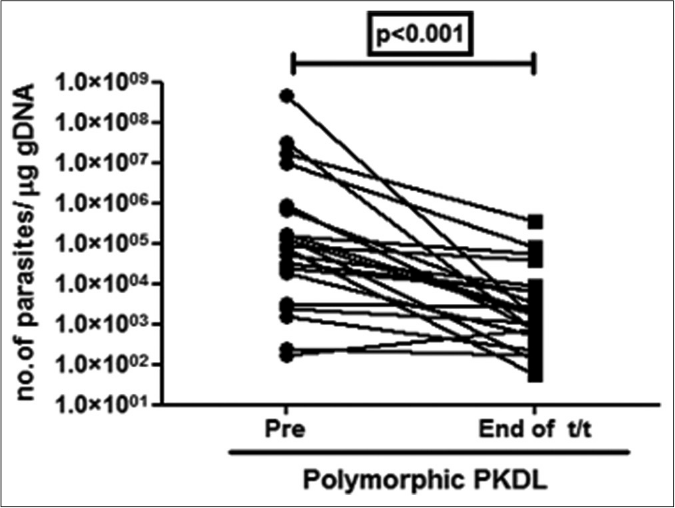
- Before-after plots (n = 21) in patients with polymorphic post kala-azar dermal leishmaniasis indicating the parasite load at disease presentation (●) and end of treatment with liposomal amphotericin b (◼)
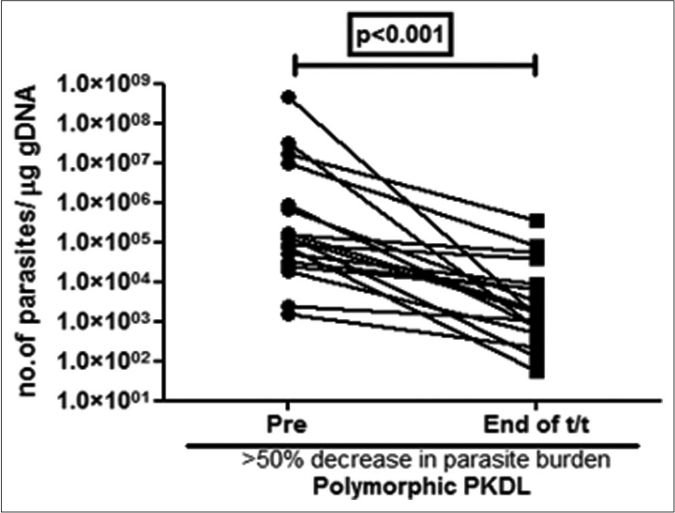
- Before-after plots (n = 18) in patients with polymorphic post kala-azar dermal leishmaniasis indicating the parasite load at disease presentation (●) and end of treatment with liposomal amphotericin b (◼) who showed >50% decrease in parasite burden
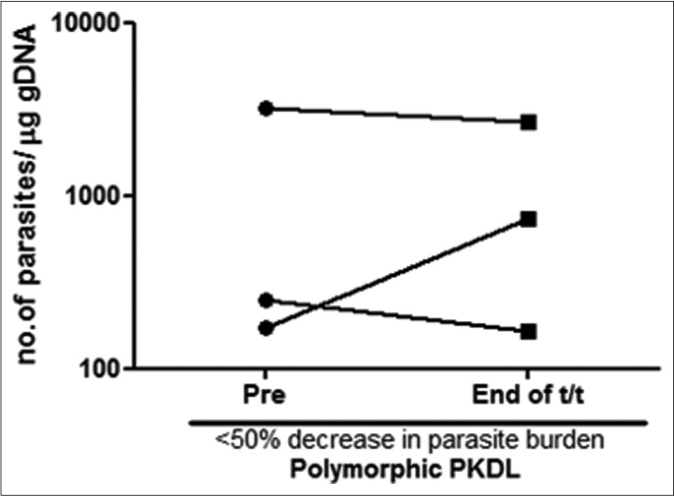
- Before-after plots (n = 3) in patients with polymorphic post kala-azar dermal leishmaniasis indicating the parasite load at disease presentation (●) and end of treatment with liposomal amphotericin b (◼) who showed <50% decrease in parasite burden
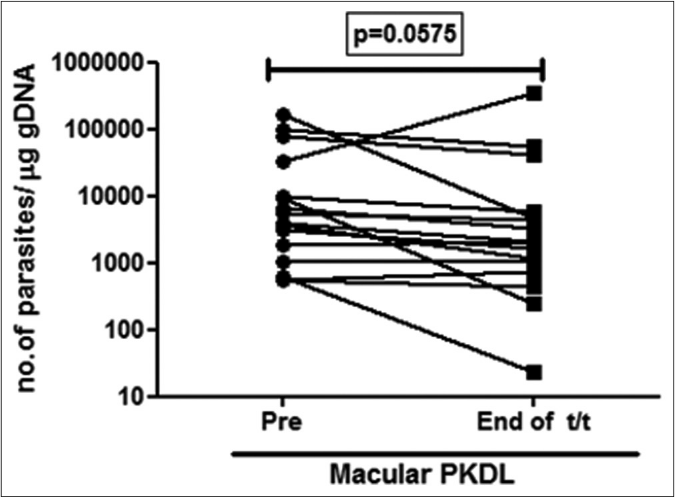
- Before-after plots (n =17) in patients with macular post kala-azar dermal leishmaniasis indicating the parasite load at disease presentation (●) and end of treatment with liposomal amphotericin b (◼)
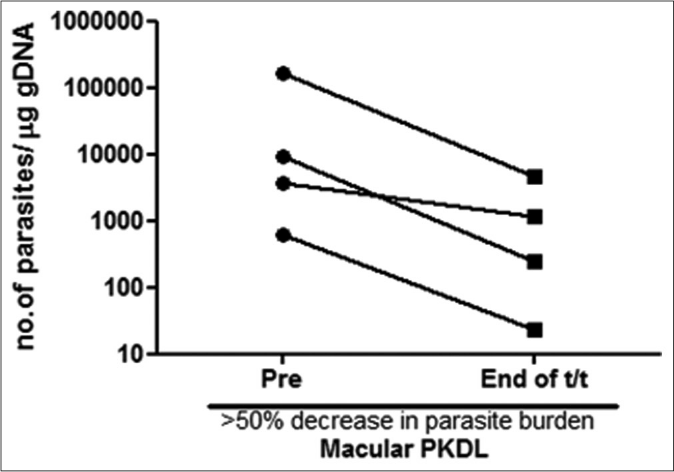
- Before-after plots (n = 4) in patients with macular post kala-azar dermal leishmaniasis indicating the parasite load at disease presentation (●) and end of treatment with liposomal amphotericin b (◼) who showed >50% decrease in parasite burden
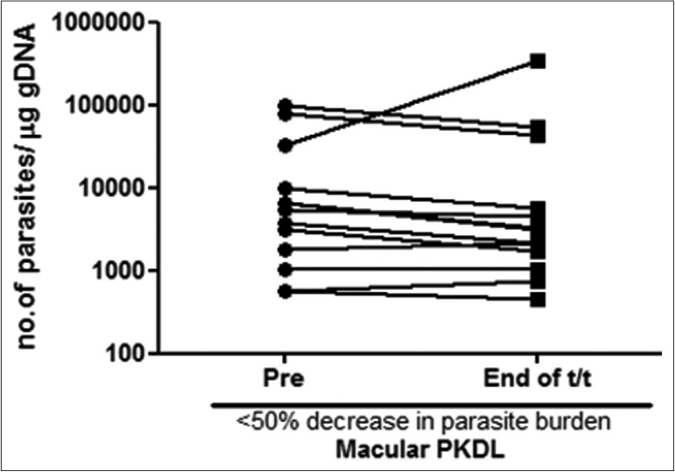
- Before-after plots (n= 13) in patients with macular post kala-azar dermal leishmaniasis indicating the parasite load at disease presentation (●) and end of treatment with liposomal amphotericin b (◼) who showed <50% decrease in parasite burden
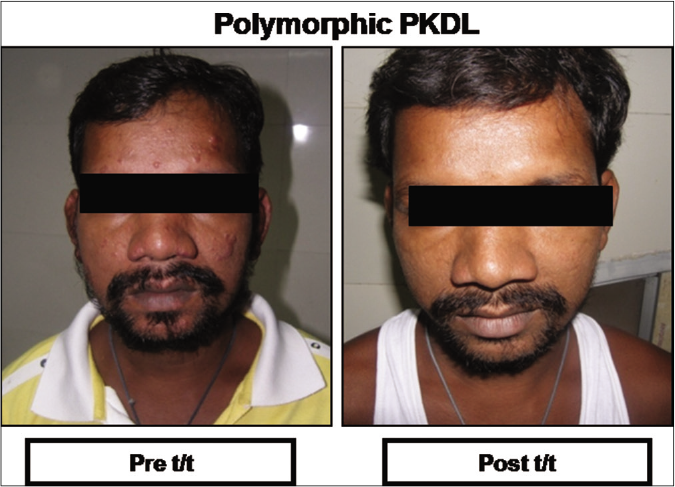
- Representative clinical features of a patient with polymorphic post kala-azar dermal leishmaniasis at disease presentation (Pre t/t) and following completion of treatment with liposomal amphotericin b (Post t/t)
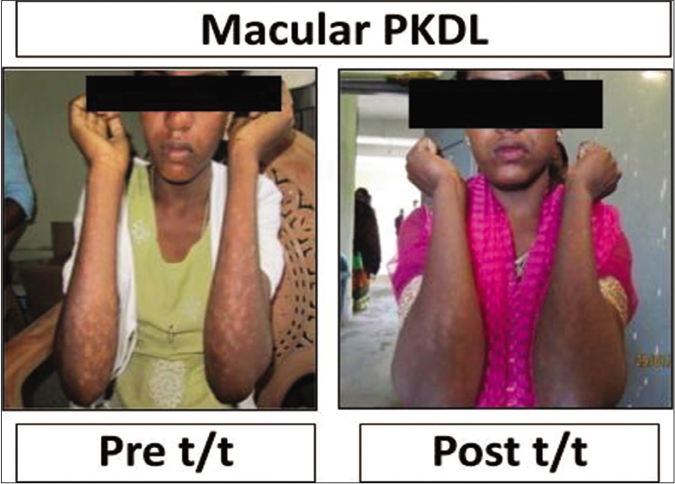
- Representative clinical features of a patient with macular post kala-azar dermal leishmaniasis at disease presentation (Pre t/t) and following completion of treatment with liposomal amphotericin b (Post t/t)
Increased presence of CD68+ macrophages was limited to polymorphic PKDL
An 18-fold increase in CD68+ cell infiltration was seen in polymorphic PKDL when compared with macular PKDL at disease presentation and healthy controls (93.4 vs. 4.6 vs 4.0 cells mm−2) [Figures 2a-b].
![Status of CD68+ cells in dermal biopsies of patients with post kala-azar dermal leishmaniasis:representative immunohistochemical profiles of a healthy control and a patient with polymorphic or macular post kala-azar dermal leishmaniasis (using horseradish peroxidase and 3,3'-diaminobenzidine as the chromogenic substrate,×100 and ×400 magnification [as indicated by white arrows])](/content/126/2022/88/2/img/IJDVL-88-201-g011.png)
- Status of CD68+ cells in dermal biopsies of patients with post kala-azar dermal leishmaniasis:representative immunohistochemical profiles of a healthy control and a patient with polymorphic or macular post kala-azar dermal leishmaniasis (using horseradish peroxidase and 3,3'-diaminobenzidine as the chromogenic substrate,×100 and ×400 magnification [as indicated by white arrows])
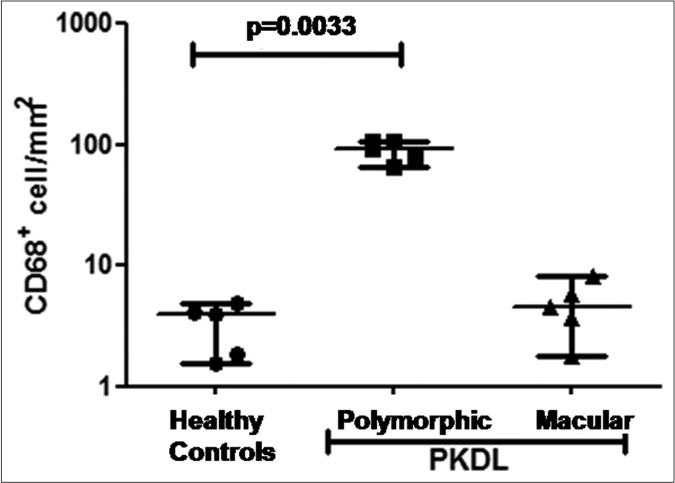
- Scatter plots showing the status of CD68+ cells in healthy controls (●, n= 5), patients with polymorphic (◼, n=5) and macular (▲, n=5) post kala-azar dermal leishmaniasis. Each horizontal bar represents the median value
Discussion
A major challenge in monitoring macular PKDL is the inability to detect Leishman Donovan bodies. However, a substantial parasite load can be measured in macular PKDL by parasite DNA detection11, although this is significantly lower than in polymorphic PKDL.8
L-AMB has proved to be a game changer in the treatment of visceral leishmaniasis12 but there is no standardized regimen for the use of this drug in PKDL and an empiric dose of 30 mg/kg bodyweight was recommended.13 The effectiveness of amphotericin B is attributed to the formation of a complex with membrane ergosterol leading to the creation of pores resulting in parasite death.14 The enhanced efficacy of the liposomal formulation is attributed to its stable intercalation within liposomes which when coated by opsonins leads to an enhanced uptake by the host macrophages in circulation or in the reticuloendothelial system.14 Therefore, the concentration of L-AMB in the lesion is dependent on the degree of macrophage infiltration, and this has been validated in a murine model of cutaneous leishmaniasis.15 In PKDL, this differential response of the two variants to L-AMB [Figures 1a-h], may be the result of the higher accumulation of the drug in polymorphic lesions, secondary to a greater degree of infiltration by CD68+ macrophages as there was an over 18-fold increase in the infiltration of CD68+ cells in polymorphic PKDL as compared to macular PKDL and healthy controls [Figures 2a and b].
A limitation of this study was the short follow-up. To assess the therapeutic response monitoring is necessary for at least 12 months. However, as the majority of our patients with PKDL belonged to the lower socio-economic strata and owing to the invasive nature of the biopsy, many patients refused or were lost to long-term follow-up.
Conclusion
This study established that the dramatic response of polymorphic PKDL to L-AMB may be due to the more intense cellular infiltration and accompanying inflammation. This suggests pharmacokinetic differences between the two variants. Therefore, in PKDL, the significant impact of the immune milieu upon pharmacokinetics is an important consideration during drug development and dosing regimens, and this scenario may be extrapolatable to diseases with an inflammatory component.
Acknowledgement
We are thankful to the Department of Health and Family Welfare, West Bengal for providing technical and personnel support during the field surveys.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This study was supported financially by Indian Council for Medical Research (Grant number: 6/9-7 [151]2017-ECD II); Dept. of Health Research, Government of India (Grant number: Dept. of Health Research/Human Resource Development, HRD/Fellowship/SUG-05/2015-16); Fund for Improvement of S and T infrastructure in Universities and Higher Educational Institutions Program, Dept. of Science and Technology, Government of India (Grant number: SR/FST/LS1-663/2016); Department of Science and Technology, Government of West Bengal (Grant number: 969 [Sanc.]/ST/P/S&T/9G-22/2016). MC is a recipient of a JC Bose Fellowship, Science Engineering and Research Board, Dept. of Science and Technology, Government of India and RS is the recipient of a Senior Research Fellowship from Indian Council for Medical Research, Government of India.
Conflicts of interest
There are no conflicts of interest.
References
- Frequently asked Questions on Visceral Leishmaniasis (Kala Azar) Available from: http://www.searo.who.int/entity/world_health_day/2014SEACD274.pdf [Last accessed on 2020 Mar 08]
- [Google Scholar]
- Visceral leishmaniasis elimination targets in India, strategies for preventing resurgence. Expert Rev Anti Infect Ther. 2018;16:805-12.
- [CrossRef] [PubMed] [Google Scholar]
- Post kala azar dermal leishmaniasis in the Indian subcontinent: A threat to the South East Asia Region Kala Azar Elimination programme. PLoSNegl Trop Dis. 2017;11:e0005877.
- [CrossRef] [PubMed] [Google Scholar]
- Active surveillance identified a neglected burden of macular cases of Post Kala azar Dermal Leishmaniasis in West Bengal. PLoSNegl Trop Dis. 2019;13:e0007249.
- [CrossRef] [PubMed] [Google Scholar]
- Biomarkers in Post kala azar dermal leishmaniasis. Front Cell Infect Microbiol. 2019;9:228.
- [CrossRef] [PubMed] [Google Scholar]
- In situ immune profile of polymorphic vs. macular Indian Post Kala azar dermal leishmaniasis. Int J Parasitol Drugs Drug Resist. 2019;11:166-76.
- [CrossRef] [PubMed] [Google Scholar]
- Monitoring of parasite kinetics in Indian Post Kala azar dermal leishmaniasis. Clin Infect Dis. 2018;66:404-10.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and effectiveness of short course ambisome in the treatment of Post Kala Azar dermal leishmaniasis: A prospective cohort study in Bangladesh. Clin Infect Dis. 2018;67:667-75.
- [CrossRef] [PubMed] [Google Scholar]
- Impaired activation of lesional CD8(+) T cells is associated with enhanced expression of Programmed Death 1 in Indian Post Kala azar Dermal Leishmaniasis. Sci Rep. 2019;9:762.
- [CrossRef] [Google Scholar]
- Histopathological characteristics of post kala azar dermal leishmaniasis: A series of 88 patients. Indian J Dermatol Venereol Leprol. 2015;81:29-34.
- [CrossRef] [PubMed] [Google Scholar]
- Single dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med. 2010;362:504-12.
- [CrossRef] [PubMed] [Google Scholar]
- National kala azar Elimination Programme. Available from: https://nvbdcpgov.in/Doc/RoadmapKA_2014.pdf. [Last accessed on 2020 Mar 19]
- [Google Scholar]
- It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front Microbiol. 2012;3:286.
- [CrossRef] [PubMed] [Google Scholar]
- Relation between skin pharmacokinetics and efficacy in ambisome treatment of murine cutaneous leishmaniasis. Antimicrob Agents Chemother. 2018;62:17.
- [CrossRef] [PubMed] [Google Scholar]






