Etiological prevalence and antifungal sensitivity patterns of dermatophytosis in India – A multicentric study
Corresponding author: Dr. Sushil Tahiliani, Dr. Tahiliani’s Clinic, A 201/202, Gasper Enclave, Ambedkar Road, Pali Market, Next to Gold’s Gym, Bandra West, Mumbai, Maharashtra, India. drsushiltt@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Tahiliani S, Saraswat A, Lahiri AK, Shah A, Hawelia D, Shah GK, et al. Etiological prevalence and antifungal sensitivity patterns of dermatophytosis in India – A multicentric study. Indian J Dermatol Venereol Leprol 2021;87:800-6.
Abstract
Background:
The prevalence of dermatophytes varies with season, geographical area, socio-economic factors and effective management strategies.
Aims:
The aim of the study was to assess the prevalence of pathogenic dermatophytes, clinical types of dermatophyte fungal infection, and in vitro antifungal drug susceptibility testing against dermatophytes.
Methods:
Three hundred and ninety five patients with dermatophytosis were enrolled from five cities (Mumbai, Delhi, Lucknow, Kolkata and Hyderabad) across India. All patients were subjected to clinical examination and investigations, including potassium hydroxide microscopy, fungal culture and antifungal drug susceptibility testing.
Results:
Trichophyton rubrum was the most common species identified (68.4%), followed by T. mentagrophytes (29.3%). Within species, T. mentagrophytes was prevalent in humid environmental conditions (Mumbai and Kolkata), whereas T. rubrum was prevalent in noncoastal areas (Delhi, Lucknow and Hyderabad). Tinea corporis (71.4%) and tinea cruris (62.0%) were the common clinical types observed. antifungal drug susceptibility testing data indicated that minimum inhibitory concentration required to inhibit the growth of 90% of organisms (MIC-90) was lowest for griseofulvin (0.25–3.0 μg/mL). Among oral antifungals, the mean MIC of itraconazole was within the range (0.84 [0.252] μg/ mL), whereas high mean MIC values were reported for terbinafine (0.05 [0.043] μg/mL). Among topical agents, lowest mean MIC values were reported for luliconazole (0.29 [0.286] μg/mL), eberconazole (0.32 [0.251]) μg/mL and amorolfine (0.60 [0.306]) μg/mL.
Limitations:
Lack of correlation between in vitro antifungal susceptibility and clinical outcome and absence of defined MIC breakpoints.
Conclusion:
T. rubrum was the most common, followed by T. mentagrophytes as an emerging/codominant fungal isolate in India. Tinea corporis was the most common clinical type of dermatophytosis. Mean MIC of terbinafine was above the reference range, while it was within the range for itraconazole; griseofulvin had the lowest mean MIC. Luliconazole presented the lowest mean MIC values across cities.
Keywords
Antifungal
coastal areas
dermatophytes
potassium hydroxide mount
susceptibility
tinea
Trichophyton
Plain Language Summary
Dermatophytosis is a prevalent problem in the Indian scenario due to the hot and humid climate. The various antifungal agents currently available for treatment are terbinafine, itraconazole, fluconazole, luliconazole, etc. This study was conducted to assess dermatophytes’ prevalence, clinical types of dermatophyte fungal infection, and in vitro antifungal drug susceptibility against dermatophytes. A total of 395 patients with dermatophytosis were enrolled from five cities across India. All patients were subjected to clinical examination and investigations. The results indicated that T. rubrum was the most common in India, followed by T. mentagrophytes. Tinea corporis was the most common clinical type of dermatophytosis. The mean minimum inhibitory concentration (MIC) of terbinafine was above the reference range, while it was within the range for itraconazole; griseofulvin had the lowest mean MIC. Luliconazole presented the lowest mean MIC values across different regions, particularly Delhi and Kolkata. Data from this study will help clinicians to choose a suitable antifungal agent during treatment.
Introduction
Dermatophytosis continues to be the most common cause of superficial fungal infection worldwide.1,2 Reports indicate that the epidemiology of dermatophytes varies among countries and even within different regions in the country.2 It is more prevalent in developing, particularly in tropical and subtropical countries like India, evidently due to the hot and humid climatic conditions.3 In addition to climatic factors, geographic location, health-care system, overcrowding, urbanization, population migration, environmental and personal hygiene culture, the prevalence of virulent species, socioeconomic conditions, individual immune system, etc., may also affect the epidemiology and incidence of dermatophyte infections.3,4
The various antifungal agents currently available in clinical use against dermatophytes are terbinafine, itraconazole, fluconazole, luliconazole, etc. Even though antifungal agents’ inappropriate use may result in resistant strains, their activity against dermatophytes has not yet been fully explored. The research outlining the antifungal susceptibility of common dermatophyte species in India is inadequate, posing a therapeutic challenge to practitioners.5 Furthermore, despite the high incidence and clinical relevance, multicentric evidence depicting the present-day clinico-epidemiological patterns of dermatophytosis across India is scarce. The magnitude of the concern thus demands studies across different geographic locations within India to increase the generalizability of the data.
Hence, considering the nature of region-wise species variability and the recent rise in antifungal therapeutic failure observed clinically, in this multicentric study, we assessed the etiological prevalence of pathogenic dermatophytes, clinical types of dermatophyte fungal infections, and in vitro antifungal drug susceptibility testing against dermatophytes to understand the variation in minimum inhibitory concentrations (MICs) levels of antifungals among dermatophytes.
Methods
In this cross-sectional, multicentric study, patients were enrolled from five different cities (Mumbai, Delhi, Kolkata, Lucknow and Hyderabad) across 13 centers in India between July 2018 and January 2019. The Royal Pune Independent Ethics Committee approved the study. All patients with dermatophyte infections visiting the outpatient department during this period were screened. A total of 395 consecutive patients aged between 18 and 65 years (~30 from each center), clinically suspected with dermatophyte skin infection (excluding infection at the sites of nails, palms, soles and scalp) with recurrent cases of tinea and other atypical presentations, receiving antifungal treatment, and willing to have minimum three days washout period before antifungal drug susceptibility testing of the clinical specimen (fungal isolate), were recruited. Patients with a non-mycotic pathology in the area of fungal infection or any condition that, in the investigator’s opinion, does not justify the patient’s inclusion in the study were excluded from the study.
All patients provided written consent in the patient authorization form to participate in the study. A detailed history was obtained from all patients, who were then subjected to clinical examinations and investigations, including a wet preparation for direct microscopic examination, fungal culture and antifungal susceptibility tests.
Sample processing
All the 395 scraping samples were collected, and the specimens were shipped to a central facility. The primary identification of dermatophytes was done using direct microscopy with 10% potassium hydroxide (KOH) mount. Direct microscopic examination of the wet-mount was performed under a microscope, under ×10 and ×40 for fungal hyphae, spores or yeast cells.
The Sabouraud dextrose agar (SDA) was used for isolation and identification of fungal isolates. Specimens were cultured on SDA media (MicroMaster Laboratories Pvt. Ltd) with 0.05% chloramphenicol alone (MicroMaster Laboratories Pvt. Ltd), or with 0.5% cycloheximide (HiMedia Laboratories Pvt. Ltd) and 0.05% chloramphenicol (MicroMaster Laboratories Pvt. Ltd) and incubated at 30°C for up to four weeks. Cultures were examined once a week and professed negative if no growth was observed until 6 weeks. Identification of dermatophytes to the species level was done by assessing the colony morphology, microscopy (Lactophenol Cotton Blue Mount), and physiological and biochemical tests. Further antifungal drug susceptibility testing was performed, and the minimum inhibitory concentration (MIC) of the drugs was determined.
Antifungal drug susceptibility testing
Antifungal drug susceptibility testing was performed as per the microbroth dilution technique of Clinical and Laboratory Standards Institute Guidelines (CLSI M38-A).6,7 The antifungal drug susceptibility testing was done for seven antifungal agents, namely, luliconazole, sertaconazole, eberconazole, itraconazole, terbinafine, griseofulvin and amorolfine. The MIC for the antifungals was interpreted according to the CLSI M38-A guidelines.
Statistical analysis
Assuming the difference in prevalence rates of dermatophyte fungal infections in various regions of India (73.3%, 64.9%, 36.6% and 58.2%), the sample sizes calculated were 301, 351, 357 and 374, respectively, for estimating the expected prevalence rate with 5% error margin and 95% confidence.8-11 Considering the lowest prevalence of 36.6%, a sample size of 357 was selected. However, taking into account the feasibility and dropout percentage of 10%, a sample size of 395 patients was selected for this study. Considering the prevalence rate of dermatophyte fungal infection as 36.6%, a sample size of 357 was selected within a 5% error margin and 95% confidence.12 Continuous variables were summarized descriptively. All statistical analyses were done using Statistical Analysis System® version 9.4 software.
Results
Male preponderance was observed (63.8%) among 395 cases studied. The mean age of the study population was 36.6 ± 13.76 years. Most patients were in the 18–30 years group (n = 165), followed by 31 to 40 years (n = 87), > 50 years (n = 76) and 41 to 50 years (n = 67). About 91.6% of patients had previous episodes of dermatophyte infection. A family history of skin diseases was reported in 82 (20.8%) patients, of which only 50 (12.7%) were on treatment.
Sample collection
In 159 (40.3%) patients, samples were collected scraping the groin skin (male: 47.6% and female: 27.3%). Other sites of sample collection (in >5% patients) were abdomen (17 [8.8%]), buttock (15 [7.7%]) and thigh (13 [6.7%]) in males and abdomen (10 [7.8%]), buttock (14[10.9%]), gluteal region (10 [7.8%]) and skin scrapings from abdomen (8[6.3%]) in females.
Clinical types
Tinea corporis was the most common presentation (282 [71.4%]), followed by tinea cruris (245 [62.0%]), tinea pedis (35 [8.9%]) and tinea capitis (25 [6.3%]) [Figure 1]. Infected sites for > 5% of patients were groin (51 [12.9%]), abdomen (28 [7.1%]) and buttock and groin (20 [5.1%]).
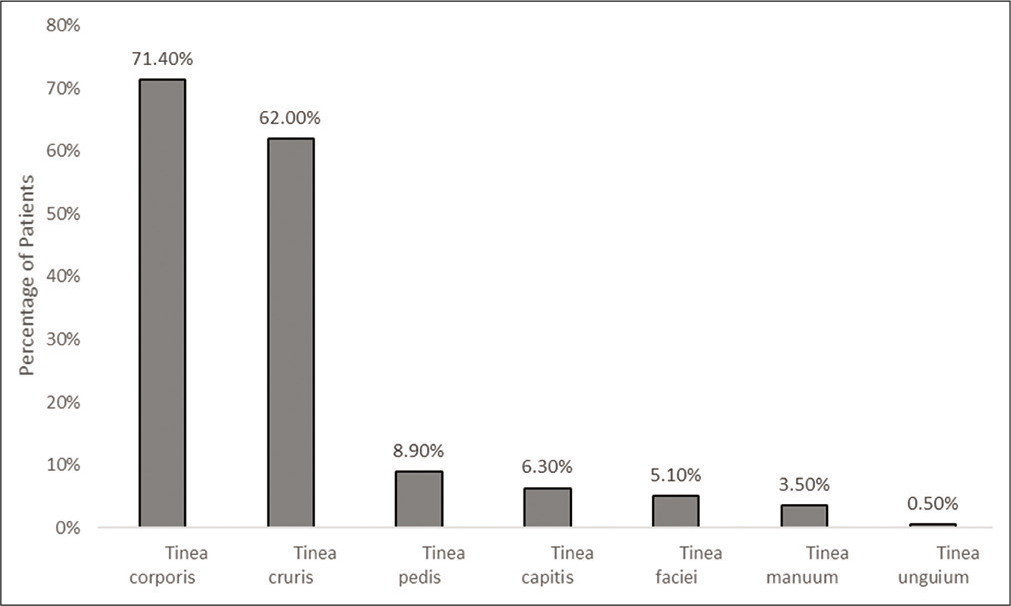
- Clinical presentation (n = 395)
Clinical features
Itching (99.0%), scaling (89.1%), dryness (80.3%) and inflammation (41.3%) were the most common clinical presentations [Table 1]. Relatively, a lesser proportion of patients presented lesion with central clearing surrounded by an advancing, red, scaly, elevated border (39.5%), erythema (36.5%) and pustules (5.3%).
| Category | Male (n=252) (%) | Female (n=143) (%) | Total (n=395) (%) |
|---|---|---|---|
| Itching | 250 (99.2) | 141 (98.6) | 391 (99.0) |
| Dryness | 195 (77.4) | 122 (85.3) | 317 (80.3) |
| Inflammation | 111 (44.0) | 52 (36.4) | 163 (41.3) |
| Scaling | 230 (91.3) | 122 (85.3) | 352 (89.1) |
| Pustules | 12 (4.8) | 9 (6.3) | 21 (5.3) |
| Erythema | 96 (38.1) | 48 (33.6) | 144 (36.5) |
| Alopecia | 8 (3.2) | 1 (0.7) | 9 (2.3) |
| Local hair loss | 4 (1.6) | 3 (2.1) | 7 (1.8) |
| Lesion with central clearing surrounded by an advancing, red, scaly and elevated border(Ring worm lesions) | 98 (38.9) | 58 (40.6) | 156 (39.5) |
| Annular patches of inflammatory or non-inflammatory alopecia | 6 (2.4) | 1 (0.7) | 7 (1.8) |
| Erythema and mild scaling on the dorsal aspect of the hands | 2 (0.8) | 1 (0.7) | 3 (0.8) |
Direct microscopy of wet mount (KOH preparation)
The results of the study were stratified based on age, gender and location. Out of 395, 349 (84.8%) were KOH positive, while 174 (44.1%) were culture positive (female: 45.5% and male: 43.3%). Trichophyton genus represented the majority of the isolates of dermatophytes. Trichophyton rubrum was the most commonly reported (120 [68.4%]), followed by T. mentagrophytes (51 [29.3%]) and T. tonsurans (2 [2.0%]) [Table 2]. Within species, T. mentagrophytes was predominant in Mumbai and Kolkata as compared to other cities (Delhi, Hyderabad and Lucknow). Furthermore, the prevalence of T. rubrum was significantly higher in patients with less than 50 years of age versus patients more than 50 years of age (P < 0.001), while the prevalence of T. mentagrophytes was higher in patients with more than 50 years of age (P < 0.001).
| Species | Gender | Age Group | ||
|---|---|---|---|---|
| Male (n=109) (%) | Female (n=65) (%) | 18–50 (n=137) (%) | >50 years (n=37) (%) | |
| Trichophyton rubrum | 77 (70.6) | 43 (64.6) | 97 (70.8) | 22 (59.5) |
| Trichophyton mentagrophytes | 30 (27.5) | 21 (32.3) | 37 (27.0) | 14 (37.8) |
| Trichophyton tonsurans | 2 (1.8%) | 0 | 1 (0.7%) | 1 (2.7%) |
| Microsporum canis | 0 | 1 (1.5%) | 1 (0.7%) | 0 |
| Trichophyton rubrum var. granulare | 0 | 1 (1.5%) | 1 (0.7%) | 0 |
The most typical clinical manifestation in culture-positive patients was the combination of tinea corporis and tinea cruris (72 [41.4%]), followed by tinea corporis (55 [31.6%]) and tinea cruris (34 [19.5%]) alone.
Antifungal susceptibility
Antifungal susceptibility testing was done for all 174 (44.1%) culture-positive patients. Griseofulvin reported the least mean MIC values, followed by luliconazole, eberconazole, sertaconazole, amorolfine and itraconazole. Out of seven antifungals agents, high MIC values were reported only for terbinafine; the mean MIC value of terbinafine (0.05 [0.043] μg/mL) was above the reference range. However, it was noted only in 20 (11.5%) out of total culture-positive patients. Out of 20, 11 patients reported T. rubrum as the fungal isolate, whereas nine reported T. mentagrophytes as the fungal isolate. The individual high MIC values were reported up to 0.256 μg/ml [range: 0.001–0.03 μg/ml]. Higher MIC values were reported for terbinafine for both T. mentagrophytes (0.256 μg/ml) and T. rubrum (0.256 μg/ml).
The MIC values for itraconazole were within the range; while griseofulvin had the lowest mean MIC (0.25–3.0 μg/mL). The MICs of itraconazole, luliconazole, amorolfine, sertaconazole and eberconazole were within the reference range [Table 3].
| Category | Culture positive (n = 174) |
|---|---|
| Terbinafine | |
| High MIC | 20 (11.5%) |
| Susceptible | 154 (88.5%) |
| MIC (µg/mL), mean (SD) | 0.05 (0.043) |
| MIC90 | 0.001–0.03 |
| Griseofulvin | |
| High MIC | 0 (0.0%) [NE] |
| Susceptible | 174 (100.0%) |
| MIC (µg/mL), mean (SD) | 0.19 (0.082) |
| MIC90 | 0.25–3.0 |
| Itraconazole | |
| High MIC | 0 (0.0%) [NE] |
| Susceptible | 174 (100.0%) |
| MIC (µg/mL), Mean (SD) | 0.84 (0.252) |
| MIC90 | 0.05–1.0 |
| Luliconazole | |
| High MIC | 0 (0.0%) [NE] |
| Susceptible | 174 (100.0%) |
| MIC (µg/mL), Mean (SD) | 0.29 (0.286) |
| MIC90 | 0.05–1.0 |
| Sertaconazole | |
| High MIC | 0 (0.0%) [NE] |
| Susceptible | 174 (100.0%) |
| MIC (µg/mL), Mean (SD) | 0.36 (0.372) |
| MIC90 | 0.05–1.0 |
| Amorolfine | |
| High MIC | 0 (0.0%) [NE] |
| Susceptible | 174 (100.0%) |
| MIC (µg/mL), Mean (SD) | 0.60 (0.306) |
| MIC90 | 0.05–1.0 |
| Eberconazole | |
| High MIC | 0 (0.0%) [NE] |
| Susceptible | 174 (100.0%) |
| MIC (µg/mL), Mean (SD) | 0.32 (0.251) |
| MIC90 | 0.05–1.0 |
NE: Not estimable
Further, drug versus fungal isolate comparison for terbinafine indicated high median values (in percentage) of MIC for T. rubrum versus . T. mentagrophytes (P = 0.0014), whereas, for itraconazole, higher median values were reported for T. mentagrophytes versus T. rubrum [P = 0.0651].
Region-wise antifungal drug susceptibility
Antifungal susceptibility was tested across India’s five different cities (Mumbai, Kolkata, Delhi, Lucknow and Hyderabad) [Figure 2]. Griseofulvin had the lowest MIC values across regions (0.19 [0.082] μg/mL). Among the topical antifungals, luliconazole showed the lowest mean MIC values across India’s different regions, particularly Delhi and Kolkata. Amorolfine also showed the least mean MIC values in Kolkata and Delhi. None of the patients from Hyderabad reported high MIC for terbinafine. The percentage of patients with high MIC for terbinafine was significantly higher from coastal areas (Mumbai and Kolkata) versus noncoastal [P = 0.0017].

- Region-wise mean minimum inhibitory concentration values
Although the MIC of itraconazole was within the range, the upper side of a higher limit was found in the majority of the patients from Hyderabad (77.3%), and lowest from Mumbai (61.3%). T. rubrum and T. mentagrophytes were the commonly reported organisms across all five regions of India. Within species, T. mentagrophytes was predominant in Mumbai and Kolkata compared to other cities (Delhi, Lucknow and Hyderabad).
Discussion
Considering the widespread prevalence of various cutaneous mycoses in a tropical country like India, primarily due to its hot and humid climate, it is essential to understand the patterns of etiology and clinical presentations. Furthermore, numerous antifungal-resistant strains causing superficial mycoses have emerged over the recent past which may be attributed to many years of underuse, overuse and misuse of antifungal medication.13,14 Hence, the development of an expound antifungal profile may help control the transmission of the infection, in turn, the impact of resistant fungal strains in the near future. The present, one of its kind multicentric studies was intended to understand the clinical manifestations of dermatophytosis, dermatophyte species distribution and their susceptibility patterns in different regions across India.
Most enrolled patients were in the age group of 18–30 years, followed by 31–40 years which agrees with India’s reported literature on dermatophytosis-centric studies.15-18 In concordance with the previous studies, male preponderance was noted.17,19 The higher incidence in young males could be attributed to their increased physical activity, predisposing them to increased sweating. The lower incidence among females seen in this study could be attributed to their hesitation to consult physicians and the financial dependence on males.
Tinea corporis (72%) and tinea cruris (62%) were the most commonly reported clinical presentations. This observation is supported by the studies of Hanumanthappa et al.20 Sharma et al.17, Agarwal et al.21 and others;22,23 however, variation in clinical manifestation is also reported in the literature.16,24 Low prevalence of tinea unguim reported in this study could be due to the fact that scrapping was not taken from the nail, resulting in less reporting of tinea unguim. Further, an increase in the prevalence of tinea facie was noted in this study compared to the recent past,22,24,25 which could be ascribed to the activities such as using common towels for the bath by all family members. Tinea infections indeed lead to personal discomfort, and antifungal treatment regimens can last for a reasonably long duration (3–6 months).26 To prevent the unnecessary usage of toxic drugs, regular surveillance of antifungal susceptibility patterns in patients should be carried out in their long-term interest.27
In this study, the commonly reported clinical features were itching, scaling, dryness, inflammation, a lesion with central clearing surrounded by an advancing, red, scaly, elevated border (ringworm lesions) and erythema, as reported by Gupta et al.28 Furthermore, in accordance with the earlier study, infected sites for more than 5% of patients were groin, abdomen and buttock and groin.28
The literature indicates that the KOH positivity rate varies between 35.6% and 88.6%, and the rate of culture positivity between 36% and 53.6% which was confirmed in our case.15,19,29,30 Shenoy et al. in 2008 showed positive results in 53% of cases by microscopy and 35% cases by culture.31 In another study in North India, KOH mount and culture showed positive results in 53.5% and 61.2% of patients.32 In our study, the KOH positivity rate was 88.4%, and the culture positivity rate was 44.1%. The inter-laboratory difference in the techniques of sampling and KOH examinations might account for the difference in microscopic and culture findings, making it essential that all the KOH negative samples should be cultured.
Trichophyton species have been a principal causative agent of dermatophytosis than the other two genera, Microsporum and Epidermophyton. In our study also, among dermatophytes, the genus Trichophyton represented the majority of the isolates of dermatophytes. T. rubrum was the most predominant fungal isolate (68.4%), as reported by other studies from India, followed by T. mentagrophytes (29.3%).33-35 This highlights the emergence of T. mentagrophytes as a codominant fungal isolate. Recent studies from India have also reported the emergence of T. mentagrophytes as the dominant36-38 or codominant fungal isolate.33-35 In our study, within species, T. mentagrophytes was predominant in coastal cities such as Mumbai and Kolkata compared to noncoastal cities (Delhi, Lucknow and Hyderabad). A similar study from India also reported a higher prevalence of T. mentagrophytes from the coastal area.39 This could be attributed to the humid climate in the coastal cities which has been indicated as an essential component for T. mentagrophytes.40 Apart from humidity, other factors such as temperature, trauma and internal factors such as host-parasite relationships, host susceptibility and immunological factors are also indicated as probable reasons for this recent shift in prevalence.36,40,41 Further, within species, the prevalence of T. rubrum was significantly higher in patients with less than 50 years of age, while the prevalence of T. mentagrophytes was higher in patients with more than 50 years of age.
Out of seven antifungal agents tested in this study, high MIC values were reported only for terbinafine. High MIC for terbinafine was reported only in 20 (11.5%) patients out of 174. The overall mean high MIC value reported for terbinafine could be attributed to high individual patients’ data of these 20 patients. Higher MIC values were noted in both T. mentagrophytes (0.256 μg/ml) and T. rubrum (0.256 μg/ml), suggesting the virulent nature of T. mentagrophytes and T. rubrum. Furthermore, 88.5% of patients had MIC within range for terbinafine. Hence, the clinician must consider the plausible reasons such as virulence potential of the infecting species, clinical type of dermatophytosis and external factors such as heat, humidity, sweating, type of clothing and the pharmacological factors such as the quality of the drug, compliance, pharmacokinetics and absorption of the drug to understand the recalcitrant infection better.42
The region-wise comparison indicated that none of the patients from Hyderabad reported high MIC value for terbinafine. The majority of the patients with high MIC with terbinafine were from coastal areas (Mumbai and Kolkata). This has been confirmed by other studies, including from different locations.43-45
Among all antifungal agents, griseofulvin reported the lowest mean MIC value. This is an encouraging trend considering the recently reported increasing clinical resistance cases to oral antifungal among Indian patients. Vardai Pai et al. had also reported lower MIC of systemic griseofulvin and topical amorolfine than fluconazole.46
All 174 (100%) patients were susceptible to griseofulvin, itraconazole, luliconazole, sertaconazole, amorolfine and eberconazole. In our study, the mean MIC of griseofulvin (range 0.25–1 μg/mL) for Delhi and Kolkata was 0.120 μg/mL, for Mumbai μg/mL and Lucknow, it was 0.240 μg/mL and for Hyderabad, it was 0.180 μg/mL. Further, newer oral drugs like itraconazole did not report high MIC in any patient. All the causative agents reported in our study were found to be susceptible to itraconazole. The median MIC (μg/ml) of itraconazole (range 0.05–1 μg/mL) for all 174 patients was 1.0 μg/ml. However, though the MIC of itraconazole was within the range, the upper side of the higher limit was found in the majority of patients from Hyderabad (77.3%). A similar trend was noted in other regions as well. This indicates the need to optimize the use of itraconazole, emphasizing on the right dose and duration of treatment, considering the present effectiveness of oral itraconazole in our routine clinical practice. Moreover, this is the last drug in the current armamentarium, and hence rational use of itraconazole is the need of the hour.
Among topical antifungals, luliconazole showed an encouraging trend in terms of lowest mean MIC values in different regions from India, Delhi and Kolkata. Similarly, sertaconazole reported the lowest mean MIC values in Hyderabad as compared to other areas. For amorolfine, the lowest mean values were reported for Kolkata, followed by Delhi and Mumbai.
These data indicate a variation in the susceptibility of fungal isolates from different regions of India which demands the need for fungal culture and antifungal susceptibility tests to identify the causative fungi and chose the effective antifungal treatment in the early stages of infection. However, clinically successful treatment does not always correlate with the MIC value of antifungals which may be illustrated by the “90–60 rule,” which states that infections due to susceptible strains respond to appropriate therapy in 90% of cases. In contrast, infections due to resistant strains respond in approximately 60% of patients.2
Limitations
We did not corroborate the in vitro antifungal susceptibility data with the clinical outcome which is a limitation of this study. Further, the lack of MIC breakpoints to categorize the isolate as susceptible, intermediate, or resistant to an antifungal agent is another limitation. Furthermore, antifungal drug susceptibility testing studies with other molecules such as ciclopirox are necessary.
Conclusion
Dermatophytosis is a prevalent problem in the Indian scenario due to the hot and humid climate and low socio-economic status. Varied etiological agents, along with regional variations, make the management of this common cutaneous condition challenging. T. rubrum was found to be the most common, and T. mentagrophytes the emerging/codominant fungal isolate in India. Tinea corporis was the most common clinical type of dermatophytosis. Mean MIC value of terbinafine was above the reference range, while it was within the range for itraconazole; griseofulvin had the lowest mean MIC. Luliconazole presented the lowest mean MIC values across different regions, particularly Delhi and Kolkata. The data generated from this study will enable clinicians to select an appropriate antifungal agent, optimizing existing oral and topical antifungal agents used in different regions of India. However, further studies on larger samples are warranted to correlate the MIC values with clinical outcomes to define the MIC breakpoints which will help adapt to therapeutic choices with high chances of success.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This study was funded by Abbott Healthcare Pvt.Ltd. All susceptibility testing was done at the central laboratory facility with Dr Miskeen’s Lab, Mumbai.
Conflicts of interest
There are no conflicts of interest.
References
- Emerging atypical and unusual presentations of dermatophytosis in India. Clin Dermatol Rev. 2017;1:12-8.
- [CrossRef] [Google Scholar]
- The great indian epidemic of superficial dermatophytosis: An appraisal. Indian J Dermatol. 2017;62:227-36.
- [Google Scholar]
- Epidemiological characterization of dermatophytes at a tertiary care hospital in Eastern Uttar Pradesh, India. Curr Med Mycol. 2019;5:1-6.
- [Google Scholar]
- The menace of chronic and recurrent dermatophytosis in India: Is the problem deeper than we perceive? Indian Dermatol Online J. 2016;7:73-6.
- [CrossRef] [PubMed] [Google Scholar]
- Management of tinea corporis, tinea cruris, and tinea pedis: A comprehensive review. Indian Dermatol Online J. 2016;7:77-86.
- [CrossRef] [PubMed] [Google Scholar]
- Interlaboratory study of quality control isolates for a broth microdilution method (modified CLSI M38-A) for testing susceptibilities of dermatophytes to antifungals. J Clin Microbiol. 2006;44:4353-6.
- [CrossRef] [PubMed] [Google Scholar]
- Susceptibility testing of dermatophytes. Curr Fungal Infect Rep. 2009;3:142-6.
- [CrossRef] [Google Scholar]
- Prevalence of dermatophytic infection and determining sensitivity of diagnostic procedures. Int J Pharm Pharm Sci. 2014;6:35-8.
- [Google Scholar]
- Clinicomycological profile of dermatophytosis in a teaching hospital. Int J Pharm Sci Invent. 2014;3:43-6.
- [Google Scholar]
- Epidemiological studies on dermatophytosis in human patients in Himachal Pradesh, India. Springerplus. 2014;3:134.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicomycological study of dermatophytosis in south India. J Lab Phys. 2015;7:84-9.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological studies on dermatophytosis in human patients in Himachal Pradesh, India. Springerplus. 2014;3:134.
- [CrossRef] [PubMed] [Google Scholar]
- Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23:253-73.
- [CrossRef] [PubMed] [Google Scholar]
- Antifungal susceptibility pattern of dermatomycosis in a tertiary care hospital of North India. Int J Res Dermatol. 2018;4:240-5.
- [CrossRef] [Google Scholar]
- Clinico-etiologic correlates of onychomycosis in Sikkim. Indian J Pathol Microbiol. 2009;52:194-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-mycological profile of dermatophytosis in Meghalaya. Int J Med Public Health. 2013;3:254-6.
- [CrossRef] [Google Scholar]
- Recurrent dermatophytosis: A rising problem in Sikkim, a Himalayan state of India. Indian J Pathol Microbiol. 2017;60:541-5.
- [CrossRef] [PubMed] [Google Scholar]
- Mycological study of dermatophytosis in rural population. Ann Biol Res. 2011;2:88-93.
- [Google Scholar]
- A clinico-mycological study of superficial mycoses from a tertiary care hospital of a North Indian town. Virol Mycol. 2014;3:135.
- [Google Scholar]
- Clinicomycological study of 150 cases of dermatophytosis in a tertiary care hospital in South India. Indian J Dermatol. 2012;57:322-3.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-mycological study of dermatophytes in a tertiary care centre in Northwest India. Indian J Dermatol Venereol Leprol. 2014;80:194.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-mycological study of dermatophytosis in Calicut. Indian J Dermatol Venereol Leprol. 2002;68:259-61.
- [Google Scholar]
- Clinico-microbiological study of dermatophytosis in a tertiary-care hospital in North Karnataka. Indian Dermatol Online J. 2016;7:264-71.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological and clinical pattern of dermatomycoses in rural India. Indian J Med Microbiol. 2015;33:134-6.
- [CrossRef] [PubMed] [Google Scholar]
- Expert consensus on the management of dermatophytosis in India (ectoderm India) BMC Dermatol. 2018;18:6.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular identification and antifungal susceptibility patterns of clinical dermatophytes following CLSI and EUCAST guidelines. J Fungi (Basel). 2017;3:17.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of dermatophytic infection and determining sensitivity of diagnostic procedures. Int J Pharm Pharm Sci. 2014;6:35-8.
- [Google Scholar]
- Clinico mycological profile of dermatophytosis in a tertiary care hospital in West Bengal-an Indian scenario. Int J Curr Microbiol Appl Sci. 2014;3:655-66.
- [Google Scholar]
- Clinical and mycological study of dermatophytosis in Jaipur (India) Int J Pharm Pharm Sci. 2012;4:215-7.
- [Google Scholar]
- Comparison of potassium hydroxide mount and mycological culture with histopathologic examination using periodic acid-Schiff staining of the nail clippings in the diagnosis of onychomycosis. Indian J Dermatol Venereol Leprol. 2008;74:226-9.
- [CrossRef] [PubMed] [Google Scholar]
- Mycological pattern of dermatomycoses in a tertiary care hospital. J Trop Med 2015 2015:157828.
- [CrossRef] [PubMed] [Google Scholar]
- Recent trends of dermatophytosis in Northeast India (Assam) and interpretation with published studies. Int J Curr Microbiol Appl Sci. 2015;4:111-20.
- [Google Scholar]
- Clinicomycological profile of dermatophytic infections at a tertiary care hospital in North India. Int J Commun Health Med Res. 2016;2:17-22.
- [CrossRef] [Google Scholar]
- Incidence and prevalence of dermatophytosis in and around Chennai, Tamilnadu, India. Int J Res Med Sci. 2016;4:695-700.
- [CrossRef] [Google Scholar]
- The current Indian epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes-a molecular study. Mycoses. 2019;62:336-56.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological studies on dermatophytosis in human patients in Himachal Pradesh, India. Springerplus. 2014;3:134.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of dermatophytosis and its spectrum in a tertiary care hospital, Kolhapur. Indian J Basic Appl Med Res. 2016;5:595-600.
- [Google Scholar]
- Clinico-etiological study of tinea corporis: Emergence of Trichophyton mentagrophytes. Int J Sci Stud. 2017;5:161-5.
- [Google Scholar]
- Population differentiation and genetic diversity of Trichophyton rubrum as revealed by highly discriminatory microsatellites. Fungal Genet Biol. 2016;95:24-9.
- [CrossRef] [PubMed] [Google Scholar]
- Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-52.
- [CrossRef] [PubMed] [Google Scholar]
- Is Antifungal resistance a cause for treatment failure in dermatophytosis: A study focused on tinea corporis and cruris from a tertiary centre? Indian Dermatol Online J. 2018;9:90-5.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the in vitro activities of newer triazoles and established antifungal agents against Trichophyton rubrum. Antimicrob Agents Chemother. 2015;59:4312-4.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of disk diffusion method and broth microdilution method for antifungal susceptibility testing of dermatophytes. Med Mycol. 2005;43:61-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of in vitro activities of 17 antifungal drugs against a panel of 20 dermatophytes by using a microdilution assay. J Clin Microbiol. 2003;41:4817-9.
- [CrossRef] [PubMed] [Google Scholar]
- Antifungal resistance in dermatology. Indian J Dermatol. 2018;63:361-8.
- [CrossRef] [PubMed] [Google Scholar]






