Translate this page into:
Semmes-Weinstein monofilament: A tool to quantify skin sensation in macular lesions for leprosy diagnosis
Corresponding author: Prof. Marco Andrey Cipriani Frade, Dermatology Division, Department of Internal Medicine, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, São Paulo, Brasil. mandrey@fmrp.usp.br
-
Received: ,
Accepted: ,
How to cite this article: Frade MAC, Rosa DJF, Bernardes-Filho F, Spencer JS, Foss NT. Semmes-Weinstein monofilament: A tool to quantify skin sensation in macular lesions for leprosy diagnosis. Indian J Dermatol Venereol Leprol 2021;87:807-15.
Abstract
Introduction:
Hypochromatic macules with altered sensitivity are the first manifestations of skin leprosy. Validation of this sensory loss assists in the confirmation of the clinical diagnosis.
Aims:
The aim of the study was to quantify the loss of sensation in leprosy lesions using the Semmes-Weinstein monofilament to strengthen the clinical diagnosis mainly of macular forms.
Methods:
Seventy-four hypochromatic macules in the macular leprosy subgroup, 27 typical borderline leprosy subgroup lesions and 49 macules of other macular dermatoses (non-leprosy group) were evaluated using the 0.05 g force Semmes-Weinstein monofilament to quantify the alteration of sensitivity within and outside of the lesions. The esthesiometric change index was established as the total number of points with altered sensation divided by the total number of tested points within the lesions to calculate the internal esthesiometric change index and outside the lesions to calculate the peripheral esthesiometric change index; these indexes were calculated for all groups. The difference (Δ) between the esthesiometric change indices of the lesional area and the adjacent skin was calculated for the leprosy and nonleprosy groups.
Results:
The percentage of points with touch sensitivity alterations within the macular and typical borderline leprosy lesions was higher in leprosy than in the non-leprosy group. The borderline and macular leprosy presented higher esthesiometric change index within injured areas than outside injured areas or in the nonleprosy group (P < 0.005). When internal esthesiometric change index values in the macular and borderline leprosy groups were higher than 0.53 and 0.5, respectively, the receiver operating characteristic curve showed 98% sensitivity and approximately 99% specificity for both groups (P < 0.0001). Regarding the difference between indices, borderline and macular leprosy had values that were higher and closer to one than in the nonleprosy group (P < 0.0001), with 100% sensitivity and 96.5% specificity for leprosy diagnosis when ΔLG was higher than 0.34. A limitation was the inability to perform a double-blind study.
Conclusion:
Semmes-Weinstein esthesiometry is a simple, useful and low-cost tool to quantify the focal alteration of cutaneous sensitivity to improve clinical leprosy diagnosis, especially for macular lesions.
Keywords
Diagnosis
esthesiometer
leprosy
Semmes-Weinstein monofilaments
sensitivity and specificity
Plain Language Summary
Leprosy is an infectious disease of skin and nerves. This study was carried out in Brazil, a country in which this infection is native. The authors proposed to use the Semmes-Weinstein monofilaments to characterize and measure the loss of sensation in skin lesions to strengthen the diagnosis of leprosy, particularly in those forms of the disease where the patches are almost imperceptible on the skin. Seventy four light coloured small patches were applied force by monofilaments to assess the alteration of sensitivity within and outside the spots. Almost all the tested points in leprosy patients showed altered touch sensation inside the small patches. In the peripheral areas of leprosy lesions, the alteration of sensation was minimum. In other skin conditions with white patches on the skin, the proportion of altered sensation points was the least. When more than half of the points inside the patches show altered sensation, the probability of correctly diagnosing leprosy is high. Semmes-Weinstein monofilaments can distinguish the areas with normal touch sensation and are able to map the areas with a typical pattern of abnormal sensation (“like islands”), strengthening leprosy diagnosis, useful in the mild presentations of the disease.
Introduction
The earliest presentation of cutaneous leprosy is often characterized by hypochromatic macules with loss of sensation and anhidrosis, and these manifestations represent multiple mononeuropathies.1,2 Although macular presentations have been described mainly in indeterminate form, these kinds of lesions can be found in all forms of leprosy.3,4 In addition, 95% of macular leprosy patients display one or more enlarged nerves, indicating that macular cutaneous manifestations do not occur only in early leprosy cases.4
To understand the pathogenesis of changes in touch sensitivity in patients with in leprosy, we should consider the evolution of the disease, facilitated by the binding and entry of Mycobacterium leprae into Schwann cells, proliferation of bacilli within the nerve, and progression to an immune response leading to nerve damage and inflammation.5 The impairment in touch sensitivity in leprosy skin lesions usually occurs concomitantly with hypochromia. During skin stress, substance P is released from sensory nerves present in the upper dermal nerve plexus, innervating the epidermis,6,7 accelerating melanogenesis and increasing melanin production. Thus, the destruction of peripheral neural fibers by M. leprae may be a causal factor for hypochromia.4
Assessments of skin sensitivity (thermal, tactile and pain sensations) in circumscribed areas (focal impairment) are important for clinicians to identify early peripheral nerve disorders in leprosy diagnosis. These changes often precede cutaneous manifestations and are characteristic of leprosy. All classic skin leprosy lesions are always accompanied by neurological impairment.8
These changes in sensation are evaluated and followed up semiquantitatively through esthesiometry using the Semmes-Weinstein monofilaments.2 The thinnest Semmes-Weinstein monofilament has a standard perceived weight of 0.05 g and is felt on most normal exposed skin areas of a healthy person.9,10
At present, clinical leprosy diagnosis has become a major challenge, mainly in the case of macular forms, because of the difficulties inherent in detecting the damage of cutaneous nerve endings using tools with different sizes and weights that give us only qualitative and doubtful data, besides being dependent on examiner expertise. To reduce errors and increase the relevance of the sensitivity test and to be able to document it visually through drawings and photos, we proposed the use of one standard device with known size and weight (0.05 g Semmes-Weinstein monofilament) to objectively quantify the use of esthesiometry for leprosy diagnosis by determining the sensitivity changes in macular skin lesions characterized by focal sensory impairment surrounded by skin with normal sensitivity (“island pattern”).
Methods
Study group characteristics
At the Dermatology and the Leprosy Outpatient Clinic of Clinics Hospital of Ribeirão Preto Medical School of the University of São Paulo, 46 new leprosy patients (3 indeterminate and 43 borderline clinical forms) were selected for inclusion in the macular leprosy subgroup three indeterminate and 43 borderline clinical forms; these patients’ skin manifestations were exclusively hypochromatic patches and were evaluated before starting multidrug therapy. In addition, 11 borderline leprosy patients presenting typical plaque lesions were selected for inclusion in the borderline leprosy subgroup. In the pleomorphic skin presentation of borderline leprosy, developed cutaneous lesions have the characteristics of annular plaques with sharp inner margins and sloping outer margins, and large plaques with a middle region of clinically normal skin within the plaques give rise to an annular or foveolar (honeycomb) appearance.11 The non-leprosy group comprised 21 vitiligo patients and nine progressive macular hypomelanosis patients. Although the difficulty of diagnosing of achromatic lesions in vitiligo is minimal, especially in people with brown skin, we included these diseases as controls with exclusively macular changes to compare with macular leprosy lesions.
Inclusion criteria
Leprosy
The enrolled subjects underwent a standardized clinical dermato-neurological examination, according to the World Health Organization guidelines. Leprosy diagnosis was made according to the finding of at least one of the following signs/symptoms: (a) Definite loss of sensitivity within a skin lesion and/or (b) thickened or enlarged peripheral nerve with a respective loss of sensitivity and/or muscle weakness.12 All leprosy diagnoses were certified by at least two dermatologists and leprologists.
All selected borderline leprosy patients had their diagnosis confirmed clinically and histopathologically with the use of bacilli by Fite-Faraco staining and/or M. leprae DNA positivity by polymerase chain reaction.
Vitiligo
Vitiligo patients were clinically diagnosed according to the Vitiligo Global Issues Consensus Conference: characterized by the presence of asymptomatic pearly-white skin macules of different sizes and shapes.13
Progressive macular hypomelanosis
Progressive macular hypomelanosis patients were selected according to the following criteria: clinically diagnosed cases of acquired, non-scaly, confluent, hypopigmented and asymptomatic macules and patches.14,15
Patients in both groups were assessed for age, sex and number and location of macules. In the leprosy group, considering that none of the classifications for leprosy include all of the clinical manifestations of leprosy, particularly those involving macular lesions, we considered the Madrid (1953) and the Indian Association of Leprology (IAL 1982) classifications.4 All patients classified as having borderline leprosy had more than one thickened peripheral nerve.4
Exclusion criteria
Exclusion criteria included individuals with other neurological or systemic diseases that result in changes in sensitivity, lepromatous leprosy patients, and/or individuals who were experiencing reactional episodes or started multidrug therapy.
The sensitivity evaluation was performed using the 0.05 g force Semmes- Weinstein monofilament within the hypochromatic macule as a test area and on adjacent normochromatic skin as the control area, following the guidelines for the sensitivity test, as described in Table 1. All patients were evaluated with no visual contact during the skin test. The respective contralateral areas were also tested, but with completely normal testing and no limitation in terms of the area to compare and calculate indexes, we did not include this data in the study. On the other hand, it is important to highlight that the internal areas were compared to the peripheral (external) areas of the same individual located in regions innervated by a given neural trunk. The evaluation of a contralateral area would imply evaluating another neural branch (interesting to evaluate symmetry) but not assessing focal changes, in circumscribed areas supplied by nerve endings restricted to a skin region within the territory of the same neural trunk, a typical neuropathic disorder of leprosy.
| 1. The patient should be in calm and silent surroundings 2. Provide a specific time for the tests aiming to ensure that both parties (patient and doctor) are concentrated during the assessment 3. Make sure that the instrument used is smaller than the area or lesion to be tested 4. Ensure in normal sensitivity areas that the patient understands the test and the type and intensity of the sensitivity to be tested initially with eyes opened and subsequently with closed eyes 5. Preferably conduct the assessment with the patient with their eyes closed, always compare between the inside of the lesion and the adjacent skin, alternately simulated(without touching), paying attention to the responses and conditioning of the patient 6. Mark with a pen small signs “−” beforehand to precisely ensure the points to be tested, making them “+” if normoaesthesia (preserved sensitivity), “0” if anaesthesia(lack of sensitivity), or keeping it “−” when hypoaesthesia (perception preserved but less intense than in normoesthetic areas). Hyperaesthesia is rare. 7. Test is diagnostic when most points of hypoaesthesia or anaesthesia are within the suspected area (s), unlike the periphery of normoaesthesia 8. At the end, make the test visually understood to the patient |
A descriptive statistical analysis was performed. A percentage evaluation was performed to analyze the frequency of points with altered sensation (anesthetic and/or hypoesthetic) and normal sensation to 0.05 g Semmes-Weinstein monofilament inside the hypochromatic macules or borderline plaque and on the surrounding skin in the three groups. Then, the esthesiometric change index was calculated considering the total number of points with altered sensation divided by the total number of points tested inside the macule or borderline plaques. This index was also calculated for peripheral normochromatic skin. Thus, the following indices were calculated: Internal and peripheral esthesiometric change index for each hypochromatic macule and borderline plaque in leprosy and for hypopigmented macules in dermatoses from the nonleprosy group [Figure 1].
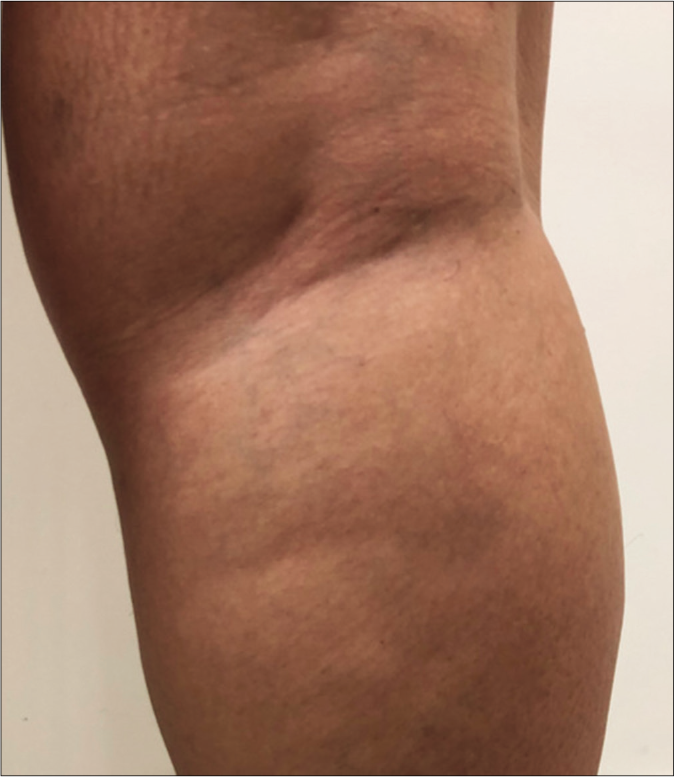
- Clinical characteristics of macular lesions in the macular leprosy subgroup (MLSG), borderline leprosy subgroup (BLSG) and nonleprosy group (NLG/vitiligo) and mapping of the touch sensation test with their respective indexes. (a) Original photo of macular hypochromatic lesion of patient of the MLSG showing the difficulties to be detected
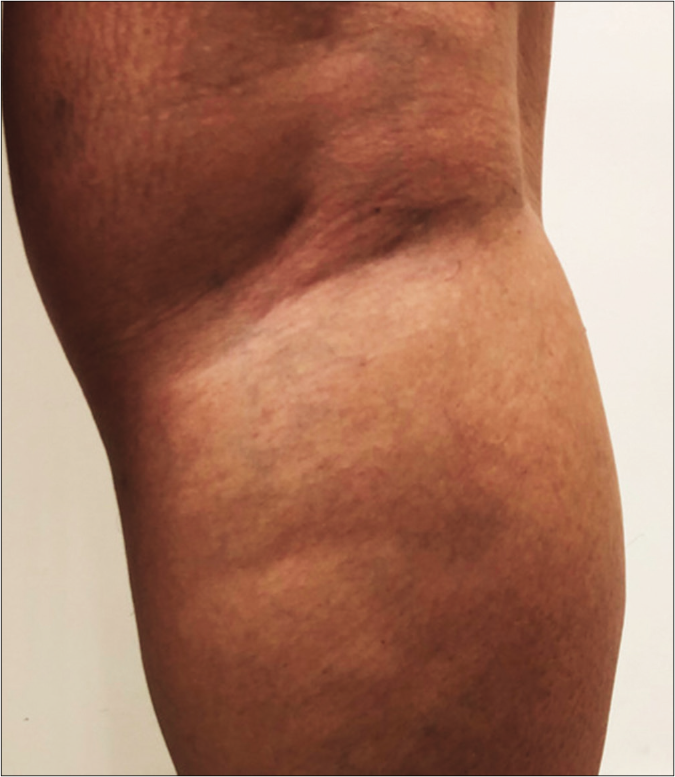
- Digital photo with 20% more contrast making the macular area more visible
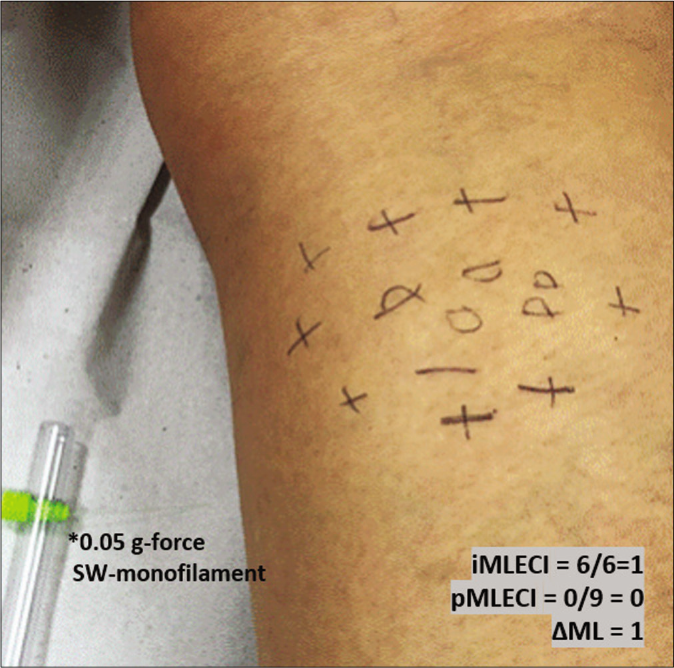
- Mapping of touch sensation test with 0.05 g force Semmes-Weinstein (SW)-monofilament in the MLSG indicating normoesthesia (+); hypoesthesia (−) and anesthesia (0) areas
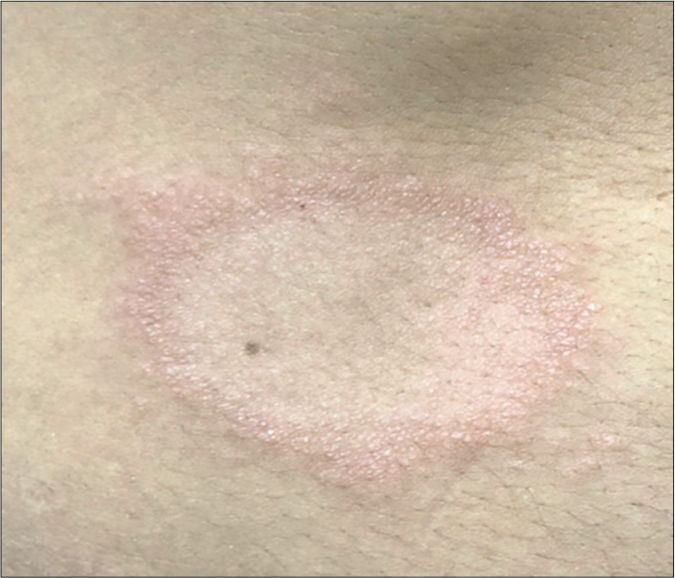
- Original photo of typical borderline lesion of patient of the BLSG
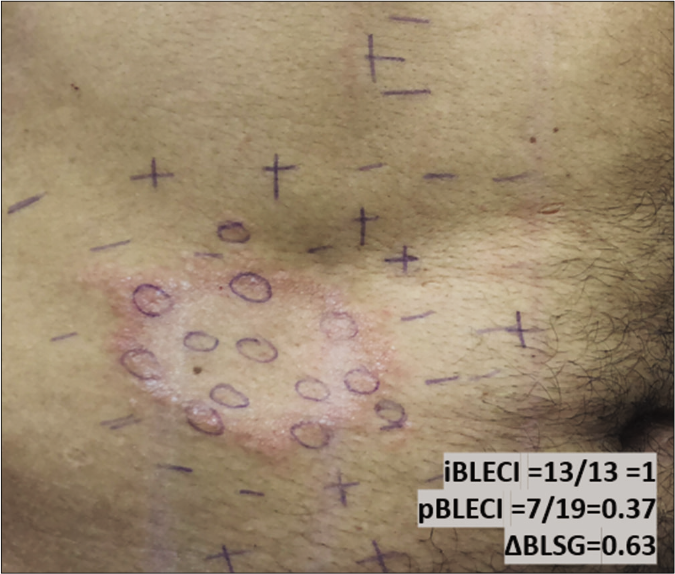
- Mapping of touch sensation test with 0.05 g force SWmonofilament in BLSG indicating normoesthesia (+); hypoesthesia (−) and anesthesia (0) areas in Borderline Leprosy subgroup (BLSG) indicating altered sensation hypoesthesia (-) or anesthesia (0) within the lesion and predominately normoesthesia (+) in peripheral areas, to calculate the (i) internal and (p) peripheral esthesiometric change indexes (ECI), and the difference (Δ) between indexes in BL subgroup.
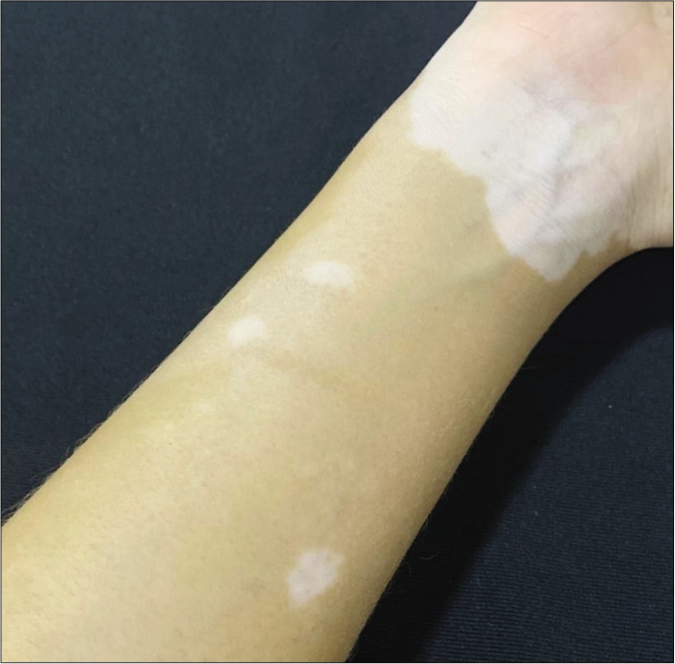
- Typical achromatic macule of vitiligo in patient in the NLG
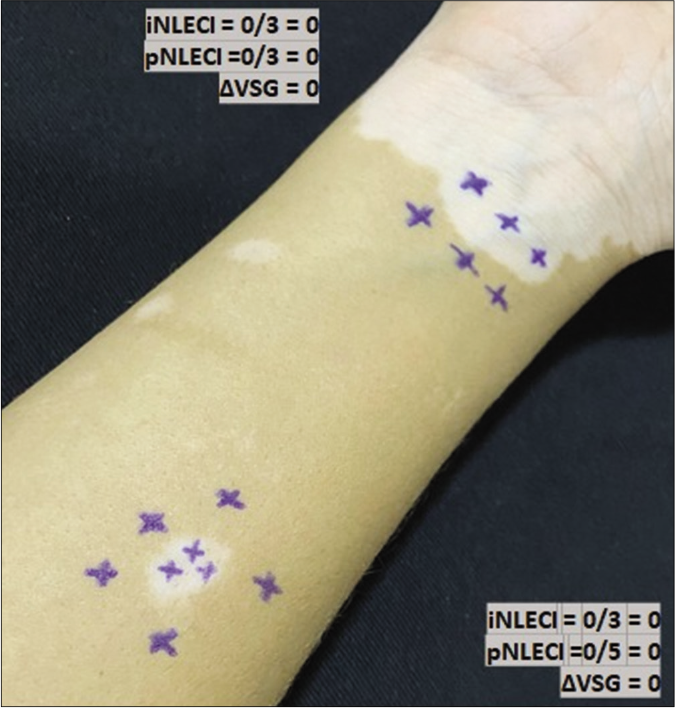
- Mapping of normal touch sensation test with 0.05 g force SWmonofilament in vitiligo lesion in the non-leprosy (NL) group indicating points with normoesthesia (+) within the lesion and in peripheral areas to calculate (i) internal and (p) peripheral esthesiometric change indexes (ECI), and the difference (Δ) between indexes in Vitiligo Subgroup (VSG).
The difference (Δ) between the esthesiometer change indexes inside the macule/plaque and outside the peripheral border of the hypopigmented area was calculated for the three groups by subtracting from the internal the peripheral index as follows: (ΔML = iML−ECI − pML−ECI), (ΔBL = iBL−ECI − pBL−ECI) and (ΔNL = iNLG−ECI − pNLG−ECI).
Statistical analysis
Data were tabulated in an Excel 2010 spreadsheet and analyzed by the statistical program GraphPad Prism 5 (San Diego CA, USA).
The results of these groups were comparatively evaluated using Student’s t test for paired samples of internal and peripheral esthesiometric change index in the same individuals, and the non-parametric Mann–Whitney test was used to test differences between different groups.
We considered the peripheral skin areas in individuals in the nonleprosy group (vitiligo and progressive macular hypomelanosis) as having healthy/normal skin sensation to calculate the sensitivity and specificity. Receiver operating characteristic curve analyses were conducted comparing three groups. The best point on the receiver operating characteristic curve was considered as the greatest value of the product between sensitivity and specificity calculated between leprosy and nonleprosy, according to Hanley and McNeil.16 The receiver operating characteristic curve showed the threshold between the rates of successes and errors to calculate the sensitivity and specificity17 of the indexes for leprosy diagnosis. The significance level was set at <5% (P < 0.05).
Ethics statement
This study was approved by the Ethics Committee of the Ribeirão Preto Clinical Hospital (Protocol number 057/2014) and followed the ethics rules for human subjects outlined by the Declaration of Helsinki. All patients enrolled in this study voluntarily read and signed an informed consent form.
Results
The demographic and clinical characteristics and total number of analyzed lesions of the leprosy and nonleprosy groups are summarized in Table 2. Except for paucibacillary patients (n = 3), all multibacillary patients had more than five lesions; however, not all lesions were evaluated to avoid the patients from becoming tired as a result of providing repeated sensitivity responses which requires a large amount of concentration. The distribution of lesion regions, the total number and the average number of points evaluated for touch sensation with the 0.05 g force Semmes-Weinstein monofilament inside (internal area) and outside (peripheral area) of the lesions in all groups are detailed in Table 3.
| Variable | LG | NLG | *P-value | ||||
|---|---|---|---|---|---|---|---|
| MLSG n=46 | BLSG n=11 | Total n=57 | VSG n=21 | PMHSG n=9 | Total n=30 | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Sex | |||||||
| Male | 21 (45.7) | 5 (45.5) | 26 (45.6) | 10 (47.6) | 0 (0) | 10 (33.3) | 0.12 |
| Female | 25 (54.3) | 6 (54.5) | 31 (54.5) | 11 (52.4) | 9 (100) | 20 (66.7) | |
| Age (years) | |||||||
| Minimum | 6 | 11 | 6 | 10 | 11 | 10 | 0.54 |
| Maximum | 88 | 68 | 88 | 72 | 33 | 72 | |
| Mean | 34.1 | 37.0 | 34.6 | 43.7 | 32.6 | 37.1 | |
| Total analyzed lesions | 74 | 27 | 101 | 28 | 21 | 49 | |
| Average | 1.6 | 2.5 | 1.8 | 1.3 | 2.3 | 1.63 | 0.58 |
| Clinical form | |||||||
| Indeterminate | 3 (6.5) | 0 | 3 (3.9) | ||||
| Borderline | 43 (93.5) | 11 (100.0) | 54 (96.1) | ||||
| WHO classification | |||||||
| Paucibacillary | 3 (6.5) | 0 | 3 (5.3) | ||||
| Multibacillary | 43 (93.5) | 11 (100.0) | 54 (94.7) | ||||
LG: Leprosy group, NLG: Nonleprosy group, MLSG: Macular leprosy subgroup, BLSG: Borderline leprosy subgroup, VSG: Vitiligo subgroup, PMHSG: Progressive macular hypomelanosis subgroup, *P: Significant difference LG×NLG (total)−X2-test for sex and Mann Whitney test for age and number of macules, WHO: World Health Organization
| Groups | LG | NLG | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MLSG | BLSG | NLG | ||||||||||
| Lesion sites | n | % | n | % | N | % | ||||||
| Head | 3 | 4.1 | 1 | 3.7 | 1 | 2.0 | ||||||
| Neck | 1 | 1.4 | 0 | 0 | 1 | 2.0 | ||||||
| Anterior trunk | 19 | 26.0 | 4 | 14.8 | 6 | 12.2 | ||||||
| Posterior trunk | 18 | 24.7 | 7 | 25.9 | 24 | 49.0 | ||||||
| Superior limbs | 13 | 17.8 | 6 | 22.2 | 8 | 16.3 | ||||||
| Inferior limbs | 19 | 26 | 9 | 33.3 | 8 | 16.3 | ||||||
| Buttocks | 0 | 0 | 0 | 0 | 1 | 2.0 | ||||||
| Total | 73 | 100 | 27 | 100 | 49 | 100 | ||||||
| Areas | i | p | i | p | i | p | ||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Total | 599 | 100.0 | 419 | 100.0 | 164 | 100.0 | 146 | 100.0 | 173 | 100.0 | 258 | 100.0 |
| Average points | 8.2 | - | 5.7 | - | 9.1 | - | 8.1 | - | 3.53 | - | 5.26 | - |
| Normoesthetic | 61 | 10.2 | 346 | 82.6 | 0 | 0 | 91 | 62.3 | 161 | 93.1 | 222 | 86.0 |
| Hypoesthetic | 168 | 28.0 | 48 | 11.5 | 27 | 16.5 | 53 | 36.3 | 10 | 5.8 | 32 | 12.4 |
| Anesthetic | 370 | 61.8 | 25 | 5.9 | 137 | 83.5 | 2 | 1.4 | 2 | 1.2 | 4 | 1.6 |
LG: Leprosy group, NLG: Non-leprosy group, MLSG: Macular leprosy subgroup, BLSG: Borderline leprosy subgroup, i: Internal; p: Peripheral
The indices of internal and peripheral esthesiometric change in individuals in the leprosy and nonleprosy groups were calculated as shown in Figures 1 and 2. Both leprosy subgroups (macular and borderline) had higher internal index values (means of 0.92 and 1.0, respectively) than peripheral esthesiometric change index values (means of 0.16 and 0.3, respectively) (P < 0.001), while in the nonleprosy group, there was no difference between the mean values of the internal and peripheral indices.
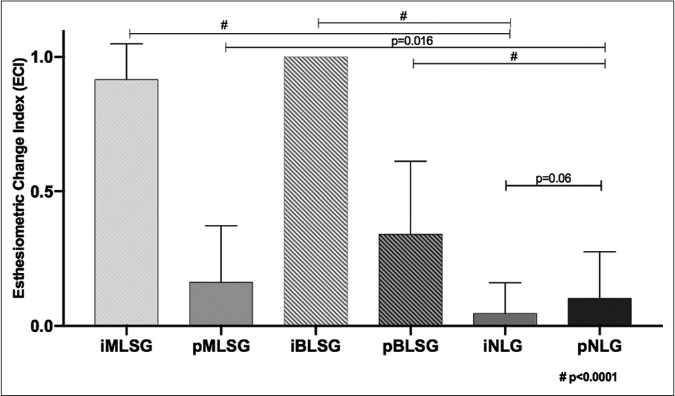
- Distribution of internal and peripheral esthesiometric change index values in leprosy (MLSG and BLSG) and difference among them. MLSG: Macular leprosy subgroup, BLSG: Borderline leprosy subgroup, NLG: Nonleprosy group
The high internal esthesiometric change index values in the macular leprosy group indicate a high occurrence of points with altered sensitivity (complete or partial loss of sensation), corresponding to focal intracutaneous nerve impairment associated with leprosy. Although the analyses of internal and peripheral indexes between macular and borderline subgroups showed significant differences, this likely reflects a difference in disease evolution being more advanced in typical borderline leprosy lesions, as demonstrated in Figure 2.
Then, the difference (Δ) between internal and peripheral esthsiometric change index in the macular leprosy (ΔMLSG), borderline leprosy (ΔBLSG) and nonleprosy groups (ΔNLG) was calculated for comparison. In each case, this value was obtained by subtracting the peripheral from the internal index for each group. The values for each participant are shown in Figure 3. The means and standard deviation of Δ in both leprosy subgroups (ΔMLSG and ΔBLSG) was similar tending to reach indexes close to 1 (0.73 ± 0.24 and 0.67 ± 0.27, respectively, P = 0.76) and significantly different (P < 0.001) from those of the nonleprosy group which exhibited a mean value close to zero (−0.06 ± 0.16), as demonstrated in Figure 3.
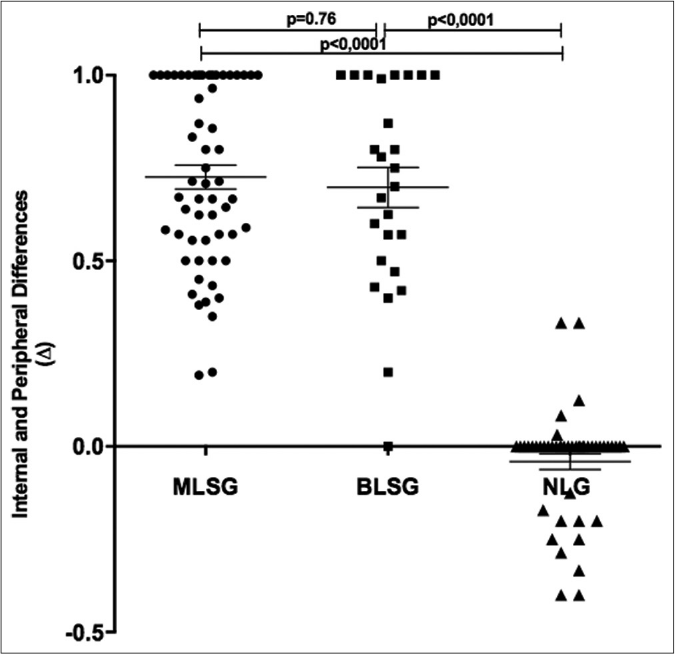
- Distribution of difference values (Δ) between internal (i) and peripheral (p) esthesiometric change index in leprosy and nonleprosy groups. ECI: Esthesiometric change index, ΔML: Difference between iMLECI and pMLECI, ΔBL: Difference between iBLECI and eBLECI, ΔNL: Difference between iNLECI and eNLECI, MLSG: Macular leprosy subgroup, BLSG: Borderline leprosy subgroup
The receiver operating characteristic curve was applied considering the peripheral areas from nonleprosy patients as the best normoesthesic control area (prompt and immediate sensation response to the test with 0.05 g monofilament) for comparison with other groups, as described in Table 4. Similarly, the internal index of the macular and borderline leprosy subgroups presented an area under the curve equal to 0.99 with 97.9% sensitivity for both and 98.6%/100% specificities when the leprosy group internal esthesiometric change index values were higher than 0.53 and 0.5, respectively (P < 0.001) [Table 5].
| Variable | LGECI | NLGECI | ||||||
|---|---|---|---|---|---|---|---|---|
| iMLSG | pMLSG | iBLSG | pBLSG | iVSG | pVSG | iPMHSG | pPMHSG | |
| Number of ECI values | 73 | 73 | 26 | 26 | 28 | 28 | 19 | 19 |
| Minimum | 0.50 | 0.00 | 0.99 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 25% Percentile | 0.85 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Median | 1.00 | 0.00 | 1.00 | 0.28 | 0.00 | 0.00 | 0.00 | 0.00 |
| 75% Percentile | 1.00 | 0.31 | 1.00 | 0.51 | 0.00 | 0.08 | 0.00 | 0.25 |
| Maximum | 1.00 | 1.00 | 1.00 | 1.00 | 0.33 | 0.33 | 0.33 | 0.50 |
| Mean | 0.92 | 0.16 | 1.00 | 0.30 | 0.03 | 0.05 | 0.04 | 0.12 |
| Std. Deviation | 0.13 | 0.21 | 0.00 | 0.28 | 0.08 | 0.10 | 0.11 | 0.17 |
| Std. Error | 0.02 | 0.02 | 0.00 | 0.05 | 0.02 | 0.02 | 0.02 | 0.04 |
| Lower 95% CI of mean | 0.88 | 0.11 | 1.00 | 0.19 | 0.00 | 0.01 | -0.02 | 0.03 |
| Upper 95% CI of mean | 0.95 | 0.21 | 1.00 | 0.41 | 0.06 | 0.09 | 0.09 | 0.20 |
ECI: Esthesiometric change indexes, LG: Leprosy group, NLG: Nonleprosy group, LGECI: Leprosy group esthesiometric change indexes, NLGECI: Nonleprosy group esthesiometric change indexes, i: Internal; p: Peripheral, MLSG: Macular leprosy subgroup, BLSG: Borderline leprosy subgroup, VSG: Vitiligo subgroup, PMHSG: Progressive macular hypomelanosis subgroup
| Group | Area under ROC curve | 95% CI | P-value | Cut off | Sensitivity % | 95% CI | Specificity% | 95% CI | Likelihood ratio |
|---|---|---|---|---|---|---|---|---|---|
| iMLECI | 0.99 | 0.9965–1.001 | <0.001 | 0.5278 | 97.87 | 88.71–99.95 | 98.63 | 92.60–99.97 | 71.45 |
| iBLECI | 0.9986 | 0.9955–1.002 | <0.001 | 0.5000 | 97.87 | 88.71–99.95 | 100.0 | 86.77–100.0 | |
| pMLECI | 0.6033 | 0.5023–0.7044 | 0.0567 | ||||||
| pBLECI | 0.7443 | 0.6178–0.8707 | <0.001 | 0.1275 | 74.47 | 59.65–86.06 | 69.23 | 48.21–85.67 | 2.42 |
| ΔMLSG | 0.9986 | 0.9955–1.002 | <0.001 | 0.3417 | 100.0 | 92.75–100.0 | 96.49 | 87.89–99.57 | 28.50 |
| ΔFLSG | 0.9823 | 0.9477–1.017 | <0.001 | 0.3667 | 100.0 | 92.75–100.0 | 92.31 | 74.87–99.05 | 13 |
ROC: Receiver operating characteristic, ECI: Esthesiometric change indexes, i: internal, p: peripheral, MLECI: Macular leprosy esthesiometric change index, BLECI: Borderline leprosy esthesiometric change index, ΔMLSG: Difference between internal and peripheral esthesiometric change index in macular leprosy subgroup, ΔFLSG: Difference between internal and peripheral esthesiometric change index in borderline leprosy subgroup
Considering the index of the peripheral area in the borderline leprosy as compared to nonleprosy group in the receiver operating characteristic curve, the sensitivity and specificity were 74.5% and 69.2%, respectively, with an area under the curve equal to 0.74 (P < 0.001), but this was not found for peripheral macular esthesiometric change index [Table 5].
The receiver operating characteristic curve analysis [Table 5] of Δ values showed 100% sensitivity and 96.5% specificity for leprosy diagnosis when the ΔML was >0.34 (P < 0.001) with an area under the curve of 0.99, while for the ΔBL, the sensitivity reached 100% and the specificity reached 92.3% for the leprosy diagnosis when the ΔBL was >0.37 (P < 0.001) with an area under the curve of 0.98.
These differences were essentially the same between the macular and borderline subgroups, showing a characteristic pattern of loss of sensation within the hypopigmented macule or in the borderline plaque in cases of more advanced disease.
Discussion
Phenolic glycolipid I of the M. leprae cell wall binds to Schwann cells in peripheral nerves, interacting with the α2 laminin chain of the axonal unit, facilitating its internalization18-20 and leading to colonization of the nerve which can lead to inflammation, nerve damage and subsequent nerve function loss.21
Electromyography has a high sensitivity and specificity in the diagnosis of peripheral neuropathy and is the gold standard for the early detection of leprosy neuropathy.2,22,23 However, there are limitations due to costs and professional qualifications and leprosy nerve impairment cannot be detected when it occurs exclusively within the intracutaneous phase. Assessment of sensitivity with Semmes-Weinstein monofilaments shows excellent agreement with electromyography findings alone2,24,25 or when combined with palpation of the peripheral nerves to detect swelling and inflammation.22
Although some studies of leprosy patient skin lesions suggest a sequential loss of thermal sensitivity, followed by loss of pain and later tactile sensation, Villarroel et al.26 examined tactile sensitivity with monofilaments and compared the results to those obtained with thermal sensitivity tests in suspected leprosy patients, finding that some individuals had a loss of tactile sensitivity with either 0.05 g force or 0.2 g force monofilaments that preceded the change in thermal sensitivity. Camargo and Baccarelli27 claimed that the change in sensitivity detected with the 0.05 g force monofilament precedes the changes in thermal and pain sensitivities. Thus, the Semmes-Weinstein monofilament has been an important tool for the timely identification of sensory loss associated with neuropathy and has also been used in monitoring and preventing disability in individuals already diagnosed with leprosy. There is not, however, a well-established description of criteria that allow for the classification of esthesiometric changes that are specifically characteristic of leprosy that would contribute to the etiological diagnosis of neuropathy from these findings.
Moreira and Alvarez9 emphasized the need for standardization of the parameters used in the sensitivity test with the esthesiometer so that the information obtained is reliable and can be reproduced. Lima and Campos28 emphasized the importance of evaluating areas that are hypoesthetic to heat, touch and pain which should be corroborated because these precede anesthesia and represent an early finding. Although the loss of sensation in a circumscribed skin area (“island pattern”) is the most important diagnostic criterion for leprosy, until now, an established quantitative method has not been proposed for subjective and qualitative sensitivity measurements.
In our leprosy patient groups, there was a predominance of points with altered sensation: About 89.8% inside the macules and 100% in the typical borderline lesions. The percentages of points with altered sensation in leprosy lesions were much higher than those found in normochromatic skin adjacent to the leprosy patch in macular (17.4%) and borderline leprosy groups (37.7%), while both of these percentages were very low within the macules of individuals not having leprosy (7%). These data reveal the significant sensitivity change in hypochromatic lesions for both macular forms and borderline lesions measured by the monofilament test. Surprisingly, the loss of sensation often extended to areas of normochromatic peripheral skin, indicating that even in the absence of visible dyschromia, early nerve damage can extend to otherwise normal appearing skin which is more common in macular leprosy patients. Considering borderline lesions as a clinical form of established disease with scattered borders, the borderline leprosy peripheral areas presented a more widely spread loss of sensation than that seen in macular forms. The lack of visible circumscription of these kinds of borderline leprosy lesions can increase the rate of error in sensory testing that can result in misdiagnosis and delayed treatment.
By performing an receiver operating characteristic curve analysis [Table 5], when the ΔML was >0.34 with an area under the curve of 0.99, we identified 100% sensitivity and 96.5% specificity for leprosy diagnosis, while the ΔBL presented 100% sensitivity and 92.3% specificity when >0.37 with an area under the curve of 0.98.
Faced with the classic applicability of Semmes-Weinstein monofilament for the detection and monitoring of touch sensation on the hand palms and foot soles semi-quantitatively in leprosy patients, it is noteworthy that the monofilament proved to be an important complementary tool that is simple to use and has a very low cost to aid in the clinical diagnosis or confirmation of leprosy, mainly in patients with negative laboratory tests, such as slit skin smear and histopathology.
With proper application and standardization of the internal and peripheral esthesiometric change index and Δ values, the use of the 0.05 g force Semmes-Weinstein monofilament was able to identify and quantify the loss of sensation within the macule and normal sensation outside the lesion (“island pattern”) with higher sensitivity and specificity than any other complementary exam for leprosy. Employing these indices would allow an earlier leprosy diagnosis and treatment that will allow for interruption of transmission and decrease the chance of lasting disability.
One limitation of our study was the lack of blinding to the diagnosis of leprosy for the researchers before the Semmes-Weinstein monofilament test and in the nonleprosy group, but all patients were blinded. Although there is no need for specific complementary tests for the diagnosis of vitiligo and progressive macular hypomelanosis, another shortcoming of the study was that we did not biopsy all patients.
Conclusion
Esthesiometry using a Semmes-Weinstein monofilament proved to be an important tool to quantify the loss of skin sensation and consequently to determine leprosy diagnosis in its macular clinical presentation. Esthesiometry using a Semmes-Weinstein monofilament is capable of easily differentiating similar-looking macules associated with other macular dermatoses, allowing us to characterize a typical pattern of areas with touch sensation change surrounded by normal sensation areas (“islands”) not following the territory of a specific nerve. The method can be easily and cheaply implemented with training to ensure reproducibility.
The values of the esthesiometric change index in leprosy patients were much higher than internal indexes in patients without leprosy, and when more than 53% of these tested points exhibited altered sensation, it enables the diagnosis of macular forms of leprosy with a sensitivity and specificity of 98 and 99%, respectively.
A Δ-value higher than 0.34, that is, an esthesiometric change index 34% higher than the peripheral change index, in macular forms allows us to infer the diagnosis of leprosy with a sensitivity of 100% and specificity of 96.5% which quantitatively defines the typical distribution of neuropathic leprosy involvement as an island pattern.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This work was supported by the Center of National Reference in Sanitary Dermatology focusing on Leprosy of Ribeirão Preto Clinical Hospital, Ribeirão Preto, São Paulo, Brazil; the Brazilian Health Ministry (MS/FAEPAFMRP-USP: 749145/ 2010 and 767202/2011); FIOCRUZ RIBEIRÃO PRETO -TED 163/2019 - Process Number 25380.102201/2019-62/ Project Fiotec: PRES-009-FIO-20.
Conflicts of interest
There are no conflicts of interest.
References
- The Weekly Epidemiological Record (WER) 2013. Global Leprosy, Update on the 2012 Situation. Geneva: World Health Organization; Available from: http://www.who.int/wer/2013/wer8835.pdf?ua=1 [Last accessed on 2014 Apr 12]
- [Google Scholar]
- Comparison of leprosy neuropathy monitoring techniques: Sensitivity test and nerve conduction study. Hansen Int. 1994;19:5-10.
- [Google Scholar]
- Clinical, Histopathological and Immunological Features of the five-type classification approved by the Indian Association of Leprologists. Lepr India. 1982;54:22-32.
- [Google Scholar]
- Macular lesions in leprosy: A clinical, bacteriological and histopathological study. J Dermatol. 1999;26:569-76.
- [CrossRef] [PubMed] [Google Scholar]
- The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-81.
- [CrossRef] [PubMed] [Google Scholar]
- Signaling pathways in melanogenesis. Int J Mol Sci. 2016;17:E1144.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms regulating melanogenesis. An Bras Dermatol. 2013;88:76-83.
- [CrossRef] [PubMed] [Google Scholar]
- Ministério da Saúde. Secretaria de Vigilância em Saúde. Hanseníase. Portal da Saúde. 2014. Brasília. Portuguese: Ministério da Saúde; Available from: http://www.portalsaude.saude.gov.br/index.php/o-ministerio/principal/secretarias/svs/hanseniase [Last accessed 2014 Apr 24]
- [Google Scholar]
- Use of Semmes-Weinstein monofilaments to assess the sensitivity of upper limbs of leprosy patients seen in the Federal District. Hansen Int. 1999;24:121-8.
- [Google Scholar]
- Manual do Usuário. Estesiômetro Sorri Kit Para Teste de Sensibilidade Cutânea [Portuguese]. Available from: http://www.sorribauru.com.br/Arquivos/Manual%20Kit%20Estesiometro.pdf [Last accessed on 2014 May 15]
- [Google Scholar]
- Portaria nº 3.125, de 07 de outubro de 2010 Aprova as Diretrizes para Vigilância, Atenção e Controle da Hanseníase. Available from: http://www.bvsms.saude.gov.br/bvs/saudelegis/gm/2010/prt3125_07_10_2010.html [Last accessed on 2017 Jun 27]
- [Google Scholar]
- Revised classification/nomenclature of vitiligo and related issues: The vitiligo global issues consensus conference. Pigment Cell Melanoma Res. 2012;25:E1-13.
- [CrossRef] [PubMed] [Google Scholar]
- Progressive macular hypomelanosis: An overview. Am J Clin Dermatol. 2007;8:13-9.
- [CrossRef] [PubMed] [Google Scholar]
- The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29-36.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of a test in medicine using a fuzzy ROC curve. Biomatemática. 2004;14:19-28.
- [Google Scholar]
- Role of the cell wall phenolic glycolipid-1 in the peripheral nerve predilection of Mycobacterium leprae. Cell. 2000;103:511-24.
- [CrossRef] [Google Scholar]
- A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science. 1983;221:1057-9.
- [CrossRef] [PubMed] [Google Scholar]
- Leprosy: An overview of pathophysiology. Interdiscip Perspect Infect Dis. 2012;2012:181089.
- [CrossRef] [PubMed] [Google Scholar]
- Hanseníase forma neural pura: Aspectos clínicos e eletroneuromiográficos dos pacientes atendidos no serviço de doenças neuromusculares do HCRP da USP no período de março de 2001 a março de 2013 [Portuguese] 2014. Dissertação (Mestrado em Neurologia) Departamento de Neurociências e Ciências do Comportamento Ribeirão Preto: Universidade de São Paulo; 2014.
- [Google Scholar]
- Sensitivity and specificity of nerve palpation, monofilament testing and voluntary muscle testing in detecting peripheral nerve abnormality, using nerve conduction studies as gold standard; a study in 357 patients. Lepr Rev. 2009;80:34-50.
- [CrossRef] [PubMed] [Google Scholar]
- New sonographic measures of peripheral nerves: A tool for the diagnosis of peripheral nerve involvement in leprosy. Mem Inst Oswaldo Cruz. 2013;108:257-62.
- [CrossRef] [PubMed] [Google Scholar]
- Leprosy neuropathy: Correlation of clinical and electrophysiological tests. Indian J Lepr. 1996;68:1-14.
- [Google Scholar]
- Reliability of Semmes Weinstein monofilament and ballpoint sensory testing, and voluntary muscle testing in Bangladesh. Lepr Rev. 1999;70:305-13.
- [CrossRef] [Google Scholar]
- Comparative study of the cutaneous sensation of leprosy-suspected lesions using Semmes-Weinstein monofilaments and quantitative thermal testing. Lepr Rev. 2007;78:102-9.
- [CrossRef] [PubMed] [Google Scholar]
- Avaliação sensitiva da neuropatia hansênica [Portuguese] In: Duerksen F, Virmond M, eds. Cirurgia Reparadora e Reabilitação em Hanseníase (1st ed). Bauru: ALM International; 1997. p. :75-84.
- [Google Scholar]
- Ministério da Educação e Saúde Departamento Nacional de Saúde. Serviço Nacional de Lepra In: Tratado de Leprologia. Rio de Janeiro: Ministério da Educação e Saúde; 1943. p. :25-152.
- [Google Scholar]






