Translate this page into:
Biologics in autoimmune bullous diseases: Current scenario
Corresponding author: Dr. Dipankar De, Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Sector 12, Chandigarh - 160012, India. dr_dipankar_de@yahoo.in
-
Received: ,
Accepted: ,
How to cite this article: Bishnoi A, De D, Handa S, Mahajan R. Biologics in autoimmune bullous diseases: Current scenario. Indian J Dermatol Venereol Leprol 2021;87:611-20.
Abstract
Autoimmune bullous diseases can be intraepidermal (pemphigus group of disorders) or subepidermal (pemphigoid group of disorders). The treatment of these disorders chiefly comprises corticosteroids and immunosuppressant adjuvants like azathioprine and mycophenolate mofetil. Autoantibodies are the main mediators of these diseases. Rituximab, a chimeric anti-CD20 monoclonal antibody targeting B-cells, has emerged as an excellent treatment option for refractory pemphigus vulgaris in the last decade. Since then, many new biologics have been proposed/explored for managing autoimmune bullous diseases. These hold potential for greater efficacy and lesser adverse effects than conventional immunosuppressants. In this review, we discuss the role of various biologics in the treatment of autoimmune bullous diseases, followed by a brief discussion on the drawbacks to their use and new developments in this area.
Keywords
Autoimmune bullous diseases
biologics
future perspectives
pemphigoid
pemphigus
Introduction
Autoimmune bullous diseases can be intraepidermal (pemphigus group of disorders) or subepidermal (pemphigoid group of disorders). The introduction of systemic corticosteroids significantly reduced mortality due to pemphigus. The treatment of these disorders is still chiefly dependent on corticosteroids with adjuvants like azathioprine and mycophenolate mofetil. However, the adverse effects resulting from prolonged administration of these drugs remain a concern.
Biologics are defined by the United States Food and Drugs Administration (USFDA) as therapeutic agents derived from any living material (microbes, plants, animals and humans), which either mimic or block the function of naturally occurring proteins. Rituximab, a chimeric monoclonal antibody that targets B-cells expressing membrane-embedded surface molecule CD20 has emerged as an excellent option for the treatment of refractory pemphigus vulgaris in the last decade.1 Subsequently, many new biologics have been proposed for managing various autoimmune bullous dermatoses. These hold the potential for greater efficacy with lesser adverse effects than conventional immunosuppressants.2 In this review, we first discuss the role of various biologics in the treatment of autoimmune bullous diseases, followed by a brief discussion on the drawbacks to their use and new developments in this area.
Therapies Targeting B-cells
CD19, 20 and 22 modulators
Though the potential contribution of T-cells has been explored in recent studies,3,4 the pathogenesis of autoimmune bullous diseases primarily involves autoantibodies targeting the inter-cellular/matrix adhesion molecules. Since the antibodies are produced by plasma cells derived from B-cells, treatments targeting B-cells are of special interest in autoimmune diseases.
Memory B-cells give rise to short-lived plasma cells that produce pathogenic autoantibodies (in contrast to the long-lived plasma cells that produce antimicrobial antibodies). These memory B-cells and plasma cells have certain surface molecules (stimulatory CD19, 20 and inhibitory CD 22) which can be modulated to modify intracellular signals, causing either depletion of B-cells or inhibiting their proliferation and differentiation [Table 1 and Figure 1].
| Therapies targeting B-cells | |
|---|---|
| Anti-CD20 | First generation- Rituximab, ofatumumab, veltuzumab, ocrelizumab Second generation- Tositumomab, obinutuzumab (inhibits stimulatory signals provided by CD20 to memory B-cells, causes depletion of memory B-cells) |
| Anti-CD19 | Blinatumomab, inebilizumab (inhibit stimulatory signals provided by CD19 to B-cells and plasma cells) |
| CD22 modulators | Epratuzumab (augments inhibitory signals provided by CD-22 to B-cells) |
| Anti-APRIL | Atacicept |
| Anti-BAFF Anti-BAFF receptor monoclonal antibody |
Belimumab VAY736 |
| Bruton tyrosine kinase inhibitors | Ibrutinib (inhibits BCR and FcγR signaling through inhibition of Bruton’s tyrosine kinase) PRN1008 PRN 473 |
| CAAR T-cells | Live T-cells that have chimeric autoantibody receptors which are composed of the autoantigen(desmoglein 3 for pemphigus) fused to the signalling domain CD137. These CAAR T-cells recognise autoantigen-specific autoreactive B-cells |
| FcRn antagonists | Efgartigimod, rozanolixizumab, SYNT001 Intravenous immunoglobulins |
| Anti-immunoglobulin E monoclonal antibodies | Omalizumab |
| Anti-complement C1s monoclonal antibody | Sutimlimab |
| IL-4 receptor antagonist | Dupilumab |
| Anti-IL-5 monoclonal antibody | Mepolizumab |
| Anti-eotaxin-1 monoclonal antibody | Bertilimumab |
| Syk inhibitors | Fostamatinib |
| IL-17 inhibitor | Ixekizumab |
| CD40-CD40L inhibition | CD40L/CD154 inhibitors |
| Polyclonal T-regulatory cells | Can halt initial sensitization of T-cells |
BCR: B cell receptor, FcγR: Receptor for Fc portion of immunoglobulin G,
CAAR: Chimeric autoantibody receptor, BAFF: B-cell activating factor,
APRIL: A proliferation-inducing ligand, Syk: Spleen tyrosine kinase, FcRn: Neonatal Fc receptor, IL: Interleukin, CD: Cluster of differentiation
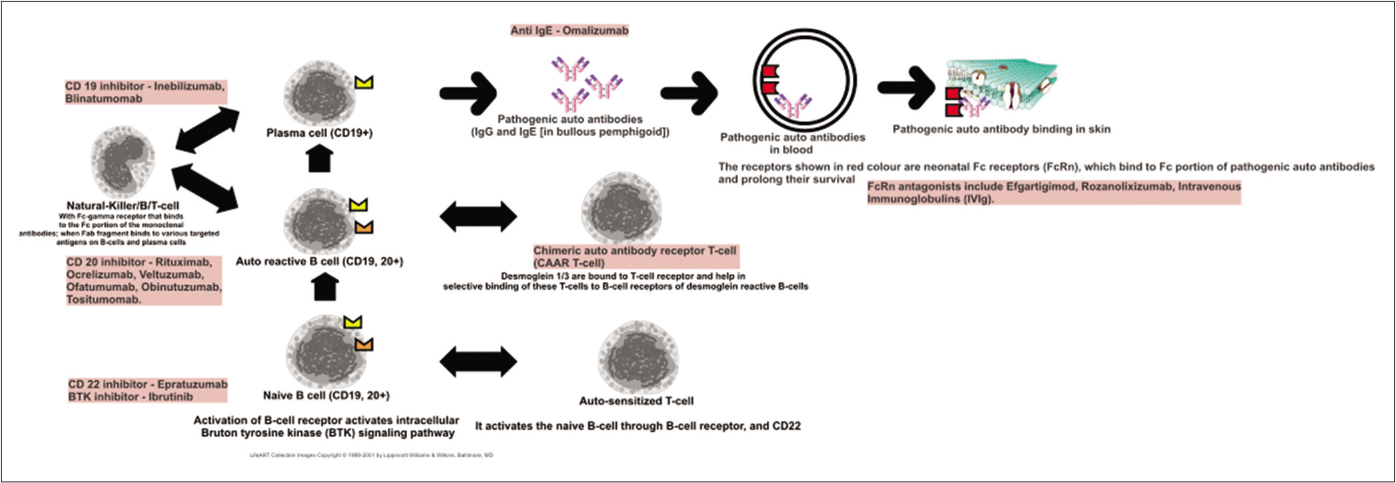
- A schematic diagram representing the pathway of B-cell sensitization, activation and production of pathogenic antibodies. Also listed are various biologics that act on different steps of this pathway
Many of these molecules were initially used in lymphomas, rheumatoid arthritis and systemic lupus erythematosus.5 Subsequently, this was extended to treat pemphigus. Some of these biologics are relatively well-known like rituximab and belimumab (human anti-B-lymphocyte stimulator monoclonal antibody) while others like epratuzumab, blinatumomab and inebilizumab are relatively new.
CD20 inhibitors are generally classified as first generation (rituximab) and second generation (ocrelizumab, ofatumumab, veltuzumab, obinutuzumab and tositumomab) on the basis of the proportion of the chimeric component in the molecule. Second generation anti-CD20 monoclonal antibodies are humanized and considered to be less immunogenic, and some of these molecules also have the advantage of subcutaneous injection. Anti-CD20 monoclonal antibodies have also been classified on the basis of their primary mechanism of action and functional differences demonstrated in vitro, as type I and II. Type I molecules (rituximab, ofatumumab, veltuzumab) cause a redistribution of CD20 into the lipid rafts, and act via both complement-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity. Type II molecules (obinutuzumab, tositumomab) do not induce complement-dependent cytotoxicity; rather, these induce a much stronger direct non-apoptotic lysosomal form of programmed cell death mediated by homotypic adhesion (two similar type of cells adhere to each other, which is followed by activation of lysosomes and release of their contents), and a lesser degree of antibody-dependent cell-mediated cytotoxicity.6
Ofatumumab, the first second-generation type I anti-CD20 monoclonal antibody, causes even more potent complement-mediated cytotoxicity than rituximab. Though reported to be successful in a recent case report,7 two therapeutic trials of ofatumumab in pemphigus were prematurely terminated due to commercial reasons (NCT01920477, NCT02613910).6 Veltuzumab is also a second generation type I anti-CD20 monoclonal antibody which results in significantly more complement-mediated cytotoxicity than rituximab; it can be administered subcutaneously. It was reported to be effective in pemphigus in a case report8 and was granted orphan drug status for pemphigus by the USFDA in 2015.6
Obinutuzumab and tositumomab are second generation type II anti-CD20 monoclonal antibodies. Though obinutuzumab has been granted a breakthrough therapy designation for adult lupus nephritis by USFDA, its use has not yet been explored in pemphigus.9
Inebilizumab and blinatumomab are anti-CD19 monoclonal antibodies that have the potential to affect both memory B-cells and plasma cells (plasma cells do not express surface CD20 and are hence not affected by rituximab). CD22 is a co-receptor of the B-cell receptor which inhibits the overactivation of the latter. Epratuzumab is a non-B-cell depleting, humanized monoclonal antibody that augments the normal inhibitory signal generated from CD22.10-12 Interestingly, epratuzumab also leads to significant reductions in the surface expression of CD19, 21 and 79b on B-cells and results in the transfer of these molecules to NK-cells and T-cells instead (trogocytosis).13 It has been tried in systemic lupus erythematosus with variable results and may be used in pemphigus in the future.14,15
Secondary impairment in T-cell function which is generally associated with B-cell depletion can explain the usual long-term disease remission induced by B-cell inhibitors, even after B-cell recovery is complete.16
B-cell activating factor/B-lymphocyte stimulator (BLyS) and a proliferation-inducing ligand (APRIL) belong to the tumor necrosis factor (TNF) superfamily of cytokines. They both support the activation, survival and proliferation of B-cells and plasma cells, and help in maintaining T-cell-independent antibody responses; they differ with respect to the distribution of their receptors.17 Their levels are positively correlated with systemic manifestations in systemic lupus erythematosus and rheumatoid arthritis. An anti-BAFF monoclonal antibody, belimumab, is approved by the USFDA for the treatment of systemic lupus erythematosus.
B-cell activating factor was found to be elevated in patients having bullous pemphigoid but not in patients having pemphigus. Moreover, its levels rose initially in the disease, followed by a rise in the levels of anti-bullous pemphigoid-180 (anti-BP180) antibodies, though the levels of both did not correlate.18 In a recent study, B-cell activating factor levels were found to be elevated in endemic Tunisian pemphigus foliaceus but not in pemphigus vulgaris.19 Further, the levels were significantly higher in those with greater body surface area involvement, recurrent active disease, and those not receiving treatment with corticosteroids.19
APRIL was found to be significantly elevated in treatment-naive pemphigus vulgaris patients as compared to patients in remission on treatment and healthy controls in one study.20 Another study however found APRIL to be elevated in patients having bullous pemphigoid early in their disease (correlated with B-cell activating factor levels) but not in pemphigus patients.21 In yet another interesting study, it was observed that giving rituximab to pemphigus patients led to a significant elevation in the levels of B-cell activating factor while APRIL and anti-pathogenic (anti-varicella zoster and anti-Ebstein Barr virus) antibodies remained unaffected; simultaneously there was a significant reduction in the levels of anti-desmoglein antibodies, suggesting a differential effect of rituximab on different B-cell populations and their proliferation ligands.22
Bruton’s tyrosine kinase inhibitors
Bruton’s tyrosine kinases are non-receptor tyrosine kinases that mediate signal transduction inside B-cells and plasma cells following the binding of activating molecules on surface B-cell receptors or Fcγ receptors. Inhibiting B-cell receptor signalling through Bruton’s tyrosine kinase inhibitors is a promising concept in the management of autoimmune bullous diseases. A patient having chronic lymphocytic leukemia with paraneoplastic pemphigus and multiorgan involvement responded favorably to ibrutinib.23 An oral molecule PRN473 (with reversible Bruton’s tyrosine kinase inhibitor activity) was found to be beneficial in canine pemphigus foliaceus.24 Another oral molecule PRN1008 showed impressive results in an open label phase II study with >50% of patients achieving disease control within four weeks of initiating treatment, and is currently undergoing a phase III clinical trial in pemphigus.25
Chimeric auto-antibody receptor T-cells
Chimeric antigen receptor T-cells were initially developed as potential antitumor therapies. These were patient-derived T-cells engineered in vitro to have tumor-antigen binding fragments of monoclonal antibodies directly fused to their signal activating machinery such as the zeta (ζ) chain of CD3, with additional linking to certain co-stimulatory molecules in the second and third generation chimeric antigen receptor T-cells, thus dissociating antigen recognition from major histocompatibility complex (MHC) restriction.26
Chimeric auto-antibody receptor T-cells were subsequently engineered as a potential treatment option for autoimmune diseases by generating chimeric immunoreceptors on their surface composed of the putative autoantigen fused to intracellular domains.27 Such T-cells are able to specifically kill the culprit B-cells via engagement of B-cell receptors. These receptors bind to the autoantigen present on the chimeric auto-antibody receptor T-cells, activating the T-cells and culminating in the killing of the pathogenic antibody-producing B-cells. Ellebrecht et al. demonstrated that chimeric auto-antibody receptor T-cells engineered with the autoantigen desmoglein-3 exhibited specific cytotoxicity against B-cells having anti-desmoglein 3 B-cell receptor in vitro. They also demonstrated in vivo in a mouse model that such chimeric auto-antibody receptor-expressing T-cells were able to expand and specifically eliminate anti-desmoglein 3 B-cell receptor-expressing B-cells.27 Notably, these T-cells did not bind free anti-desmoglein antibodies. Since these T-cells were able to form memory T-cells, their activity against the pathogenic B-cells should be persistent.27 Moreover, to manage a possible epitope spreading, these chimeric auto-antibody receptor-expressing T-cells could also be engineered to express both desmoglein 1 and 3 on their surface.
Therapies Targeting Pathogenic Autoantibodies
Neonatal Fc receptor antagonists
Targeting the neonatal Fc receptor (FcRn, Brambell receptor) forms the basis of the development of many new therapeutics.28 This receptor is encoded by the Fcgrt gene and it resembles an MHC type 1 molecule. It binds to the constant fragment (Fc) of immunoglobulin G (IgG) antibodies leading to their transport inside the cell (transcytosis), thus preventing their degradation and prolonging their half-life significantly.29 It is strongly expressed on the placenta and helps in transfer of maternal antibodies to the fetal compartment. As a corollary, these neonatal Fc receptors can also bind to pathogenic IgG antibodies resulting in increased half-lives and decreased catabolism of such antibodies; neonatal Fc receptors are therefore implicated in autoimmune diseases. Keratinocytes express neonatal Fc receptors, and knock-out mice lacking these receptors did not develop acantholysis even after passive transfer of anti-desmoglein antibodies.16
The potential of neonatal Fc receptors to prolong the half-life of antibodies has been therapeutically exploited to shorten the half-life of pathogenic ones.30 Efgartigimod (neonatal Fc receptor antagonist) is an engineered Fc fraction of immunoglobulin G1(IgG1) which saturates the neonatal Fc receptors, and therefore causes rapid degradation and clearance of pathogenic antibodies.31 The result here is similar to that with other methods that remove pathogenic antibodies, like immunoadsorption and plasma exchange but this method is novel.32 The action is specifically on IgG. No serious adverse effects viz. increased infection risk etc. were noted in healthy volunteers. The lack of infections noted with neonatal Fc receptor-antagonists might be explained by the fact that they can only reduce IgG levels by about 75% whilst sparing other types of antibodies. It might be noted here that the saturation of FcRn and the resulting rapid clearance of antibodies is considered one of the most important therapeutic mechanisms of intravenous immunoglobulins (IVIg). Efgartigimod has already been used successfully in myasthenia gravis33 and a phase two study has found encouraging results of it's use in six patients of mild to moderate pemphigus (disease control achieved in three patients in one week and in one patient in four weeks; two patients had progression of disease).32
Rozanolixizumab is a monoclonal antibody developed against neonatal Fc receptors and has shown promising IgG-reducing properties in healthy volunteers.32
Omalizumab
IgG autoantibodies against bullous pemphigoid antigen 2 (BPAg2, BP180) have long been known to be pathogenic. Recently focus has also been directed at the presence of IgE autoantibodies directed against BP180.34,35 It was seen in a recent study that these IgE-BP180 antibodies did not increase the sensitivity of diagnosis of bullous pemphigoid but their levels had a significant correlation with disease activity.36 A meta-analysis concluded that severity of bullous pemphigoid was correlated with serum IgE levels, though the disease phenotype was not.37
Omalizumab binds to the circulating IgE levels, and thus decreases the free IgE levels, thereby decreasing the IgE bound to cell surfaces (basophils, mast cells and eosinophils), which in turn decreases the expression of the receptor for Fc portion of IgE on the said cells (FcεR1). There are multiple case reports and two small case series that have demonstrated the efficacy of omalizumab in bullous pemphigoid.38,39 A systematic review has noted an 84% complete response rate with omalizumab, though a relapse rate of 80% was also noted after a mean interval of 3.2 months.40 Interestingly, there are reports of patients with rituximab-refractory bullous pemphigoid responding to omalizumab and vice versa, indicating the intricacies of the types of autoantibodies, their specific pathogenic actions and wide inherent inter-individual variations in this disease.41-43 Omalizumab has also been used successfully in the treatment of infantile bullous pemphigoid.44
Inhibiting Cytokines, Chemokines and Complements
Dupilumab, bertilimumab and mepolizumab
The presence of eosinophils in the dermis and subepidermal clefts is considered a hallmark of bullous pemphigoid. Peripheral blood eosinophilia is observed in 5-43% of patients with bullous pemphigoid.45 Activated eosinophils have been shown to cause splitting at the dermoepidermal junction by releasing eotaxin-1 and other granular contents in the presence of pathogenic autoantibodies.45 Eosinophilic infiltration in the skin is mediated by IL-4 and IL-5, the cytokines found elevated in the skin and sera of patients with bullous pemphigoid.45
Phase two trials studying IL-5 antagonist mepolizumab and anti-eotaxin-1 antibody bertilimumab in bullous pemphigoid have been completed. Mepolizumab failed to control the disease, whereas bertilimumab had an appreciable steroid-sparing effect and resulted in 84% reduction in disease severity.46 Dupilumab, an IL-4 receptor antagonist, was also reported to be effective in recalcitrant bullous pemphigoid in a case report.47
Complement activation plays an important role in the pathogenesis of bullous pemphigoid. Sutimlimab, an anti-C1s monoclonal antibody, is being evaluated for its effectiveness in bullous pemphigoid.46
Miscellaneous
Other potential targets in bullous pemphigoid include the chemoattractant receptor-homologous molecule, a prostaglandin D2 receptor which is thought to promote activation and recruitment of Th2 cells and eosinophils;45 and the Th-17 pathway involving IL-17.46 An ongoing trial is assessing the effect of ixekizumab, a humanized IL-17A antagonist, in bullous pemphigoid. Spleen tyrosine kinase and leukotriene-B4 inhibition are also potential therapeutic targets in pemphigoid diseases.25 In fact, spleen tyrosine kinase has emerged as an important non-receptor tyrosine kinase mediating inflammation in an in vivo mouse model of epidermolysis bullosa acquisita,48 and the same was demonstrated in a whole-genome analysis of the mouse skin in an experimental model of epidermolysis bullosa acquisita.49 Spleen tyrosine kinase inhibitors (like fostamatinib) could perhaps be employed in the treatment of the pemphigoid group of disorders, especially those with mechano-bullous characteristics.48
In the complex milieu of T and B-cells in pemphigus, T-cell sensitization initiates and propagates antibody production.50 This Th-cell and B-lymphocyte cross-talk primarily occurs through CD40-CD40L (CD154), and can be modulated.51 A previous study assessed the role of CD40L blocker, and concluded that blocking CD40L could prevent the formation of pathogenic antibodies in a mouse model of pemphigus.52 Polyclonal T-regulatory cells can be employed to inhibit autoreactive T-helper cells and B-lymphocytes, and phase I trials in pemphigus are ongoing to assess the potential of polyclonal T-reg administration.25
It has been suggested that the generous collection of desmoglein-specific B-cells in the skin in pemphigus renders significant interactions amongst multiple cell types including IL-21 and IL-17A producing T-cells. IL-21 activates Janus-associated kinases (JAKs) JAK1 and JAK3. A possible therapeutic role of tofacitinib (pan-JAK inhibitor) in pemphigus has been recently proposed.53
Current Status of Biologics in Pemphigus and the Pemphigoid Group of Disorders
Pemphigus group of disorders
Meta-analyses have established the efficacy of rituximab in pemphigus.54,55 Though initially used in pemphigus refractory to conventional treatments, a recent prospective study has established the efficacy of rituximab in newly diagnosed moderate to severe pemphigus as a first-line treatment, in combination with a short duration of prednisolone.56 Not only was the efficacy better, but the cumulative dose of corticosteroids and adverse effects were also significantly lower in the combination group (rituximab plus short duration prednisolone) than in the monotherapy (conventional doses of prednisolone alone) group.57 Rituximab was approved by the USFDA for pemphigus vulgaris in 2018,58 and an international panel of experts recommended it as a first-line treatment in moderate to severe pemphigus.59 It has also been used successfully in childhood and juvenile pemphigus.60 Interestingly, though pemphigus herpetiformis is an IgG-mediated disease, it did not respond to rituximab in a retrospective study.61
Ahmed and Shetty, in their meta-analysis, showed that significant clinical control was achieved within six weeks in 90–95% of pemphigus patients treated with rituximab plus low-dose corticosteroids or other immunosuppressive agents, with complete remission being achieved in 3–4 months on treatment.55 They concluded that patients receiving rituximab as per the rheumatoid arthritis protocol were more likely to achieve complete remission and remain off treatment post-rituximab.55 The largest retrospective Indian study that included 146 patients treated with a uniform rheumatoid arthritis protocol showed that complete remission off treatment could be achieved in 73.3% of the patients in 6.6 months, which was sustained for 9.1 months before relapse. Relapse was seen in 76.5% of patients.62 Time taken to achieve remission was significantly longer in pemphigus foliaceus than in pemphigus vulgaris. Another retrospective review by Heelan et al. demonstrated rituximab used in rheumatoid arthritis protocol to be a cost-effective treatment in comparison to high dose IVIg in pemphigus.63,64
Pemphigoid group of disorders
Overall, rituximab has been found to be beneficial in the pemphigoid group of disorders, though its efficacy therein is generally lower and it takes longer to achieve complete remission than in the pemphigus group of disorders. The efficacy also seems better in bullous pemphigoid than in mucous membrane pemphigoid or epidermolysis bullosa acquisita. A retrospective study reporting the outcomes in 28 patients (8 with bullous pemphigoid, 14 mucous membrane pemphigoid, 5 epidermolysis bullosa acquisita and one with linear IgA dermatosis) who received rituximab on day 1 and 15 (500 mg each in six patients and 1 g each in 22 patients) observed disease control, partial remission and complete remission in 67.9%, 57.1% and 21.4% of the patients at 14.5, 34.2 and 59.2 weeks respectively.65
Combination with intravenous immunoglobulins
Combination treatment with rituximab and intravenous immunoglobulins was employed in 12 patients of severe refractory bullous pemphigoid (mean age, 68 years) in a study.66 Clinical remission on treatment was achieved in 4.6 months and all previous treatments could be stopped in 6.2 months. Patients were followed for a mean of 73.8 months without any adverse effects being noted. Only two patients relapsed, and their disease responded to repeat rituximab infusions.
Comparison with omalizumab
A recent systematic review and meta-analysis of the use of omalizumab and rituximab in bullous pemphigoid (84 patients; 62 treated with rituximab and 22 with omalizumab) concluded that both were equally safe and efficacious, with complete response rates of 84% and 85% respectively, and adverse effects in 20% and 24% respectively. However patients treated with rituximab had significantly lower rates of recurrence (29% with rituximab vs 80% with omalizumab) and a significantly longer disease-free period until relapse occurred (10.2 months with rituximab vs 3.4 months with omalizumab).40 This is expected based on how the drugs work. However, omalizumab may be better suited for elderly patients with bullous pemphigoid having comorbidities including diabetes mellitus and other immunocompromised states.
Biosimilars
Despite the undeniable efficacy of biologics, their cost has always remained a concern; this has led to the development of 'biosimilars'. A biosimilar, though not an exact replica of the original biologic molecule, is considerably similar to the latter, with comparable efficacy and adverse effect profile. Differences arise because of inherent variations in the cell lines and purification systems used, and post-translational modifications thereafter. Biosimilars of rituximab have been used in India with good results.67
Pitfalls of Current Treatment Strategies with Rituximab
Although encouraging, the use of biologics, especially rituximab, in pemphigus and the pemphigoid group of disorders is currently associated with certain shortfalls that include the following:
Resistance and treatment failure
Relapse
Lack of clarity on optimal dosing
Infections
Route of administration
Resistance and treatment failure
Resistance to rituximab can be primary or secondary. Secondary resistance refers to reduced efficacy after initial successful infusions.68 The following factors have been postulated to cause resistance to rituximab. Many of these are well-studied in malignancies (where multiple infusions of rituximab are usually required) but not in pemphigus.
Apart from inducing hypersensitivity infusion reactions, the murine component in rituximab might also be associated with the formation of human anti-chimeric antibodies that may adversely affect the outcome of subsequent infusions by neutralizing the administered rituximab.16
Most therapeutic monoclonal antibodies are of the IgG type. The Fc fragment of these antibodies binds with Fcγ receptors present on macrophages, dendritic cells, B-cells and NK-cells (maximum expression is seen on the cells of innate immune system) while the Fab fragment binds to CD20 expressed on B-cells. This interaction leads to the neutralization of the target opsonized by the IgG (antibody-dependent cell-mediated cytotoxicity). Fcγ receptors have a multitude of actions, usually stimulatory but sometimes inhibitory (in humans, FcγRI, FcγRIIa [CD32a], FcγRIIc [CD32c], FcγRIIIa [CD16a] and FcγRIIIb[CD16b] are stimulatory, while FcγRIIb[CD32b] is inhibitory).69,70 Stimulatory Fcγ receptors activate the killing of opsonized targeted cells by macrophages or NK-cells whereas inhibitory Fcγ receptors inhibit this process. Polymorphisms in these receptors could affect antibody-dependent cell-mediated cytotoxicity and therefore outcomes of rituximab infusions. Such polymorphisms might be responsible for the primary rituximab resistance in some patients.71-73 However, polymorphisms in FcγRIIIa or FcγRIIa did not influence treatment outcomes in patients having follicular lymphoma treated with rituximab in one study.74 Overall, findings in this area have been inconsistent,74 and more studies regarding these molecules are needed, especially in pemphigus.75
Type I anti-CD20 monoclonal antibodies like rituximab have been shown to undergo internalization in the phagocytosing cells along with the CD20 molecule (a process known as trogocytosis) mediated by Fcγ receptors.68 Due to a reduction in the number of available CD20 receptors, this phenomenon can decrease the efficacy of subsequent infusions of CD20 inhibitors, and in fact has been associated with reduced survival and treatment response in patients with hematologic malignancies.68 How trogocytosis is relevant to an autoimmune disease such as pemphigus needs to be studied in detail.76
Another reason for resistance to rituximab could be long-lived plasmablasts (lacking CD20) producing anti-desmoglein antibodies. These cells can be effectively targeted using anti-CD19 antibodies, like blinatumomab and inebilizumab.16
-
Site-specific factors may be important in determining the response to rituximab. Clinical experience has suggested that despite appreciable response to rituximab elsewhere on the body, some patients have persistent lesions at a few sites such as the scalp and oral cavity. It would be of interest to look at the factors responsible for relative rituximab resistance at these poorly responding sites. Some clinical factors determining the persistence of lesions in oral cavity have been described before including the presence of deep/ crateriform ulcers, presence of the lesions on the retromolar trigone or the line of occlusion of the buccal mucosa, longer duration of disease and presence of lichenoid hue.77 It has been observed that the expression of desmoglein 3 is substantial in the scalp and buccal mucosa; this could possibly lead to a persistent immune response and treatment-refractory lesions at these sites.78
Further, it has been seen that addition of autologous serum to rituximab helped significantly in attaining anti-tumor response in central nervous system lymphomas, probably due to the addition of complement at a site normally deficient in immunocytes and complement.79
Though neonatal Fc receptors (FcRn) are known to potentiate the half-life of pathogenic autoantibodies, these receptors can also bind to administered monoclonal antibodies increasing their half-life. It remains to be seen if deactivating mutations in the Fcgrt gene encoding FcRn can cause an increased catabolism of administered monoclonal antibodies and therapeutic failure in some individuals.
CD20 transcript variants with less inherent binding to rituximab have been proposed to explain rituximab resistance in B-cell malignancies. Though these variants could not be found in pemphigus and rheumatoid arthritis patients, future research may reveal novel CD20 transcript variants that could impart primary rituximab resistance.80,81
Treatment failure and early relapse after rituximab have been associated with prior prolonged treatment with conventional immunosuppressives.82 Late introduction of rituximab for management might mean more pathogenic B-cell clones have had time to develop.82
A new subset of B-cells that produce interleukin-10 (also known as B-regulatory cells) and negatively regulate autoimmunity has been recently described. It is worth noting that the depletion of these B-regulatory cells (along with significant depletion of pathogenic B-cells) after treatment with biologics can cause a paradoxical flare of the autoimmune process.5
Relapse after rituximab therapy and factors determining it
Positive anti-desmoglein 3 titres and direct immunofluorescence are associated with an increased risk of relapse in pemphigus.83 Apart from these, factors specifically related to an early relapse after treatment with rituximab have been elucidated in a few studies. One study demonstrated a higher relapse rate in patients who had a longer duration of disease and received rituximab after being refractory to conventional treatment agents than those who received it as the first-line agent.84 However, no effect was seen on relapse rates in another study where rituximab was administered to immunosuppressant-naive and immunosuppressant-refractory patients, but the rates of complete remission off treatment were higher in immunosuppressant-naive patients receiving rituximab, even after adjusting for disease duration.85 Yet another study demonstrated an increased relapse rate and a reduced rate of complete remission in patients receiving rituximab as per the low-dose rheumatoid arthritis protocol (500 mg, 2 doses, 15 days apart) and those with a longer duration of disease. This study also demonstrated reduced relapses in patients receiving plasma exchange and immunoadsorption.86
Choosing between ultra-low, low and high dose protocols of rituximab
In pemphigus, rituximab was initially employed in lymphoma protocol (375 mg/m2). This was followed by reports of a successful outcome with low-dose protocols (1g or 500mg, 15 days apart).87 A meta-analysis by Wang et al. showed that there were no differences in remission and relapse rates between high (≥2000 mg/ cycle) and low dose (<1500 mg/ cycle) protocols, or between rheumatoid arthritis and lymphoma protocols, but the remission lasted longer with high-dose protocols.54 Another study showed no difference in the remission rates on the standard rheumatoid arthritis protocol (1 g, 2 doses, 15 days apart) and the lymphoma protocol, but the low-dose rheumatoid arthritis protocol (500 mg, 2 doses, 15 days apart) was associated with reduced rates of complete remission.85 A more recent study also associated the lymphoma protocol of rituximab and older age with the attainment of complete remission off treatment in pemphigus.88
Since the burden of autoreactive B-cells in pemphigus is significantly less than that of neoplastic B-cells in hematologic malignancies, it seems prudent to employ lower doses of rituximab in pemphigus.89 A recent study has demonstrated 68%, 74% and 97% reductions of B-cell counts in healthy volunteers following 0.1, 0.3 and 1 mg/m2 dosage of rituximab respectively. B-cell recovery was 60% in the 1 mg/m2 group at four weeks and almost complete in the 0.1 mg/m2 and 0.3 mg/m2 groups at the same time.90 This finding in healthy volunteers should pave the way for well-designed clinical trials assessing the efficacy of ultra-low-dose rituximab in patients of pemphigus. By extrapolating the data of this study, the authors have proposed a regimen of 100 mg rituximab, two doses, three months apart, to be sufficient to cause B-cell depletion for six months. This approach apart from being cost-friendly is also attractive owing to a probably lesser resultant immunosuppression.89
Infections
Though clinical experience suggests that rituximab is remarkably safe with infusion reactions being the commonest reported complications, infections, late-onset neutropenia and septicemia after rituximab infusions can sometimes be life-threatening, especially with the high-dose protocols.91 Therefore, some precautions should be routinely followed, including screening for hepatitis B and C, HIV and uncontrolled diabetes; up to date vaccinations before starting rituximab; and prophylaxis for Pneumocystis carinii pneumonitis after rituximab infusion.92 Guidelines strongly recommend prophylactic immunization with non-live vaccines for patients of autoimmune bullous disorders in whom biologic therapies are planned.93
High-dose intravenous immunoglobulins (IVIg) have been successfully used in severe pemphigus.94-96 Ahmed et al. have reported a protocol combining rituximab and IVIg with no infectious complications or loss of efficacy in ten patients. They proposed that the loss of antibodies (pathogenic autoantibodies and normal immunoglobulins) brought about by rituximab is compensated by the addition of nonpathogenic normal immunoglobulin in IVIg, when using these in combination.97
Routes of administration
Rituximab is traditionally administered intravenously. Intravenous administration requires the patients to stay in the hospital for a longer time. Subcutaneous route, apart from being simpler, is less painful and requires a shorter stay in the hospital. Subcutaneous rituximab, manufactured using recombinant hyaluronidase, was non-inferior to the intravenous form in studies performed for various lymphomas, better tolerated by patients and was approved by the USFDA in 2017 for the same.98 Many of the second generation CD20 inhibitors have the advantage of subcutaneous administration which would result in better patient tolerance and less time consumption for patients and office staff alike.
Intralesional administration of rituximab in a dose of 5 mg/ cm2 (2 such doses, 15 days apart) has also been attempted by Vinay et al. and found to be beneficial. This minimal dosage was also associated with an almost complete decline in CD19 cells in the blood.99
Future
An optimal regimen of rituximab for autoimmune bullous disorders needs to be established. The benefits, hazards and cost-effectiveness of combination treatments (especially rituximab with intravenous immunoglobulins, omalizumab and other immunosuppressants) need to be studied. The future might also lie in further exploring the genetic bases and therapeutic modulation of antibody checkpoints (FcγRs), including prediction of treatment failure or resistance in those harbouring inhibitory polymorphisms. Research is also ongoing regarding the efficacy of new molecules inhibiting downstream pathways in acantholysis, like p38 mitogen-activated protein kinase (MAPK) inhibitors, and signal transducers and activators (STAT3) inhibitors.100 Much translational research is under way and the future of biologics in autoimmune bullous dermatoses looks bright.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Shifting focus in the therapeutics of immunobullous disease. Indian J Dermatol. 2017;62:282-90.
- [Google Scholar]
- Targeted therapies for autoimmune bullous diseases: Current status. Drugs. 2018;78:1527-48.
- [CrossRef] [Google Scholar]
- Autoreactive T cells in the immune pathogenesis of pemphigus vulgaris. Exp Dermatol. 2013;22:699-704.
- [CrossRef] [Google Scholar]
- Assessment of the effects of rituximab monotherapy on different subsets of circulating T-regulatory cells and clinical disease severity in severe pemphigus vulgaris. Dermatology. 2016;232:572-7.
- [CrossRef] [Google Scholar]
- B cell modulation strategies in autoimmune diseases: New concepts. Front Immunol. 2018;9:622.
- [CrossRef] [Google Scholar]
- Next-generation anti-CD20 monoclonal antibodies in autoimmune disease treatment. Auto Immun Highlights. 2017;8:12.
- [CrossRef] [Google Scholar]
- Successful treatment of pemphigus vulgaris withofatumumab. J Drugs Dermatol. 2018;17:1338-9.
- [Google Scholar]
- Subcutaneous veltuzumab, a humanized anti-CD20 antibody, in the treatment of refractory pemphigus vulgaris. JAMA Dermatol. 2014;150:1331-5.
- [CrossRef] [Google Scholar]
- New insights in Type I and II CD20 antibody mechanisms-of-action with a panel of novel CD20 antibodies. Br J Haematol. 2018;180:808-20.
- [CrossRef] [Google Scholar]
- Epratuzumab for systemic lupus erythematosus. Expert Opin Biol Ther. 2014;14:1045-7.
- [CrossRef] [Google Scholar]
- The mechanistic impact of CD22 engagement with epratuzumab on B cell function: Implications for the treatment of systemic lupus erythematosus. Autoimmun Rev. 2015;14:1079-86.
- [CrossRef] [Google Scholar]
- Epratuzumab targeting of CD22 affects adhesion molecule expression and migration of B-cells in systemic lupus erythematosus. Arthritis Res Ther. 2010;12:R204.
- [Google Scholar]
- Trogocytosis of multiple B-cell surface markers by CD22 targeting with epratuzumab. Blood. 2013;122:3020-9.
- [CrossRef] [Google Scholar]
- Efficacy and safety of epratuzumab in patients with moderate/ severe active systemic lupus erythematosus: Results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann Rheum Dis. 2014;73:183-90.
- [CrossRef] [Google Scholar]
- Efficacy and safety of epratuzumab in moderately to severely active systemic lupus erythematosus: Results from two phase III randomized, double-blind, placebo-controlled trials. Arthritis Rheumatol. 2017;69:362-75.
- [CrossRef] [Google Scholar]
- Pemphigus: Current and future therapeutic strategies. Front Immunol. 2019;10:1418.
- [CrossRef] [Google Scholar]
- The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282-9.
- [CrossRef] [Google Scholar]
- Serum levels of BAFF are increased in bullous pemphigoid but not in pemphigus vulgaris. Br J Dermatol. 2006;155:330-6.
- [CrossRef] [Google Scholar]
- High B-cell-activating factor levels in endemic Tunisian pemphigus. Indian J Dermatol Venereol Leprol. 2017;83:496-9.
- [CrossRef] [Google Scholar]
- Assessment of serum a proliferation-induced ligand level in patients with pemphigus vulgaris. J Egypt Womens Dermatol Soc. 2018;15:122-6.
- [CrossRef] [Google Scholar]
- Increased serum levels of a proliferation-inducing ligand in patients with bullous pemphigoid. J Dermatol Sci. 2007;46:53-60.
- [CrossRef] [Google Scholar]
- B-cell-directed therapy for inflammatory skin diseases. J Invest Dermatol. 2009;129:289-301.
- [CrossRef] [Google Scholar]
- Successful use of Bruton's kinase inhibitor, ibrutinib, to control paraneoplastic pemphigus in a patient with paraneoplastic autoimmune multiorgan syndrome and chronic lymphocytic leukaemia. Australas J Dermatol. 2017;58:e240-2.
- [Google Scholar]
- A new treatment for autoimmune blistering diseases-The efficacy of the Bruton's tyrosine kinase inhibitor PRN473 in canine pemphigus foliaceus. J Am Acad Dermatol. 2016;74:AB141.
- [Google Scholar]
- Current clinical trials in pemphigus and pemphigoid. Front Immunol. 2019;10:978.
- [CrossRef] [Google Scholar]
- Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin Biol Ther. 2011;11:855-73.
- [CrossRef] [Google Scholar]
- Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353:179-84.
- [CrossRef] [Google Scholar]
- Neonatal Fc receptor: From immunity to therapeutics. J Clin Immunol. 2010;30:777-89.
- [CrossRef] [Google Scholar]
- The neonatal Fc receptor as therapeutic target in IgG-mediated autoimmune diseases. Cell Mol Life Sci. 2010;67:2533-50.
- [CrossRef] [Google Scholar]
- Biological therapy of autoimmune blistering diseases. Expert Opin Biol Ther. 2019;19:149-56.
- [CrossRef] [Google Scholar]
- Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Invest. 2018;128:4372-86.
- [CrossRef] [Google Scholar]
- Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology. 2019;92:e2661-73.
- [Google Scholar]
- IgE-mediated mechanisms in bullous pemphigoid and other autoimmune bullous diseases. Expert Rev Clin Immunol. 2016;12:267-77.
- [CrossRef] [Google Scholar]
- Role of IgE in bullous pemphigoid: A review and rationale for IgE directed therapies. G Ital Dermatol Venereol. 2012;147:251-7.
- [Google Scholar]
- Correlation of serum levels of IgE autoantibodies against BP180 with bullous pemphigoid disease activity. JAMA Dermatol. 2017;153:30-8.
- [CrossRef] [Google Scholar]
- IgE autoantibodies and their association with the disease activity and phenotype in bullous pemphigoid: A systematic review. Arch Dermatol Res. 2018;310:11-28.
- [CrossRef] [Google Scholar]
- Omalizumab therapy for bullous pemphigoid. J Am Acad Dermatol. 2014;71:468-74.
- [CrossRef] [Google Scholar]
- Successful treatment of bullous pemphigoid with omalizumab. Arch Dermatol. 2012;148:1241-3.
- [CrossRef] [Google Scholar]
- Rituximab and omalizumab for the treatment of bullous pemphigoid: A systematic review of the literature. Am J Clin Dermatol. 2019;20:209-16.
- [CrossRef] [Google Scholar]
- Successful treatment of a bullous pemphigoid patient with rituximab who was refractory to corticosteroid and omalizumab treatments. Case Rep Dermatol. 2017;9:38-44.
- [CrossRef] [Google Scholar]
- Dramatic exacerbation of bullous pemphigoid following rituximab and successful treatment with omalizumab. Eur J Dermatol. 2019;29:213-5.
- [Google Scholar]
- IgE blockade in autoimmunity: Omalizumab induced remission of bullous pemphigoid. Clin Immunol. 2019;198:54-6.
- [CrossRef] [Google Scholar]
- Successful management of severe infant bullous pemphigoid with omalizumab. Br J Dermatol. 2012;166:1140-2.
- [CrossRef] [Google Scholar]
- Eosinophils as putative therapeutic targets in bullous pemphigoid. Exp Dermatol. 2017;26:1187-92.
- [CrossRef] [Google Scholar]
- Pharmacological advances in pemphigoid. Curr Opin Pharmacol. 2019;46:34-43.
- [CrossRef] [Google Scholar]
- Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. 2018;154:1225-6.
- [CrossRef] [Google Scholar]
- The Syk tyrosine kinase is required for skin inflammation in an in vivo mouse model of epidermolysis bullosa acquisita. J Invest Dermatol. 2017;137:2131-9.
- [CrossRef] [Google Scholar]
- Whole-genome expression profiling in skin reveals Syk as a key regulator of inflammation in experimental epidermolysis bullosa acquisita. Front Immunol. 2018;9:249.
- [CrossRef] [Google Scholar]
- Skin barrier and autoimmunity-mechanisms and novel therapeutic approaches for autoimmune blistering diseases of the skin. Front Immunol. 2019;10:1089.
- [CrossRef] [Google Scholar]
- Emerging treatment options for the management of pemphigus vulgaris. Ther Clin Risk Manag. 2018;14:757-78.
- [CrossRef] [Google Scholar]
- Tolerance induction by the blockade of CD40/CD154 interaction in pemphigus vulgaris mouse model. J Invest Dermatol. 2006;126:105-13.
- [CrossRef] [Google Scholar]
- Tofacitinib as the potent treatment for refractory pemphigus: A possible alternative treatment for pemphigus. Dermatol Ther. 2018;31:e12696.
- [Google Scholar]
- Efficacy of rituximab for pemphigus: A systematic review and meta-analysis of different regimens. Acta Derm Venereol. 2015;95:928-32.
- [CrossRef] [Google Scholar]
- A comprehensive analysis of treatment outcomes in patients with pemphigus vulgaris treated with rituximab. Autoimmun Rev. 2015;14:323-31.
- [CrossRef] [Google Scholar]
- First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): A prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-40.
- [CrossRef] [Google Scholar]
- Treatment update of autoimmune blistering diseases. Dermatol Clin. 2019;37:215-28.
- [CrossRef] [Google Scholar]
- Diagnosis and management of pemphigus: recommendations by an international panel of experts. J Am Acad Dermatol. 2020;82:575-585.e1.
- [Google Scholar]
- Successful use of rituximab in the treatment of childhood and juvenile pemphigus. J Am Acad Dermatol. 2014;71:669-75.
- [CrossRef] [Google Scholar]
- Pemphigus herpetiformis: A case series and review of the literature. Int J Dermatol. 2015;54:1014-22.
- [CrossRef] [Google Scholar]
- Effectiveness and safety analysis of rituximab in 146 Indian pemphigus patients: A retrospective single-center review of up to 68 months follow-up. Indian J Dermatol Venereol Leprol. 2020;86:39-44.
- [CrossRef] [Google Scholar]
- Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol. 2014;150:703-8.
- [CrossRef] [Google Scholar]
- Cost and resource use of pemphigus and pemphigoid disorders pre-and post-rituximab. J Cutan Med Surg. 2015;19:274-82.
- [CrossRef] [Google Scholar]
- Effectiveness and safety of rituximab in recalcitrant pemphigoid diseases. Front Immunol. 2018;9:248.
- [CrossRef] [Google Scholar]
- Treatment of recalcitrant bullous pemphigoid (BP) with a novel protocol: A retrospective study with a 6-year follow-up. J Am Acad Dermatol. 2016;74:700-8.e3.
- [Google Scholar]
- Therapeutic potential of biosimilars in dermatology. Indian J Dermatol Venereol Leprol. 2015;81:451-6.
- [CrossRef] [Google Scholar]
- The need for markers and predictors of Rituximab treatment resistance. Exp Dermatol. 2014;23:236-7.
- [CrossRef] [Google Scholar]
- Activatory and inhibitory Fcγ receptors augment rituximab-mediated internalization of CD20 independent of signaling via the cytoplasmic domain. J Biol Chem. 2015;290:5424-37.
- [CrossRef] [Google Scholar]
- Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol. 2014;15:707-16.
- [CrossRef] [Google Scholar]
- Targeting FcγRs to treat antibody-dependent autoimmunity. Autoimmun Rev. 2016;15:510-2.
- [CrossRef] [Google Scholar]
- Targeting the antibody checkpoints to enhance cancer immunotherapy-focus on FcγRIIB. Front Immunol. 2019;10:481.
- [CrossRef] [Google Scholar]
- Impact of Fc gamma-receptor polymorphisms on the response to rituximab treatment in children and adolescents with mature B cell lymphoma/leukemia. Ann Hematol. 2016;95:1503-12.
- [CrossRef] [Google Scholar]
- Genetic variation in low-to-medium-affinity Fcγ receptors: Functional consequences, disease associations, and opportunities for personalized medicine. Front Immunol. 2019;10:2237.
- [CrossRef] [Google Scholar]
- Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood. 2011;118:2530-40.
- [CrossRef] [Google Scholar]
- Identification of factors associated with treatment refractoriness of oral lesions in pemphigus vulgaris. Br J Dermatol. 2017;177:1583-9.
- [CrossRef] [Google Scholar]
- Regional variation in the expression of pemphigus foliaceus, pemphigus erythematosus, and pemphigus vulgaris antigens in human skin. J Invest Dermatol. 1991;96:159-61.
- [CrossRef] [Google Scholar]
- Treatment of primary central nervous system lymphoma with induction of complement-dependent cytotoxicity by intraventricular administration of autologous-serum-supplemented rituximab. Cancer Sci. 2006;97:80-3.
- [CrossRef] [Google Scholar]
- The alternative CD20 transcript variant is not a surrogate marker for resistance to rituximab in patients with rheumatoid arthritis. Rheumatology (Oxford). 2015;54:1744-5.
- [CrossRef] [Google Scholar]
- Lack of expression of an alternative CD20 transcript variant in circulating B cells from patients with pemphigus. Exp Dermatol. 2014;23:66-7.
- [CrossRef] [Google Scholar]
- Rituximab in pemphigus: Road covered and challenges ahead. Indian Dermatol Online J. 2018;9:367-72.
- [Google Scholar]
- Immunologic prediction of relapse in patients with pemphigus vulgaris (PV) in clinical remission. J Am Acad Dermatol. 2016;74:1160-5.
- [CrossRef] [Google Scholar]
- Rituximab as first-line adjuvant therapy for pemphigus: Retrospective analysis of long-term outcomes at a single center. J Am Acad Dermatol. 2018;78:806-8.
- [CrossRef] [Google Scholar]
- An assessment of treatment history and its association with clinical outcomes and relapse in 155 pemphigus patients with response to a single cycle of rituximab. J Eur Acad Dermatol Venereol. 2015;29:777-82.
- [CrossRef] [Google Scholar]
- Low-dose rituximab is effective in pemphigus. Br J Dermatol. 2012;166:405-12.
- [CrossRef] [Google Scholar]
- Factors associated with complete remission after rituximab therapy for pemphigus. JAMA Dermatol. 2019;155:1404-09.
- [CrossRef] [Google Scholar]
- Ultra-low dosage regimen of rituximab in autoimmune blistering skin conditions. Front Immunol. 2018;9:810.
- [CrossRef] [Google Scholar]
- Single, very low rituximab doses in healthy volunteers-A pilot and a randomized trial: Implications for dosing and biosimilarity testing. Sci Rep. 2018;8:124.
- [CrossRef] [Google Scholar]
- Infectious complications of rituximab therapy in renal disease. Clin Kidney J. 2017;10:455-60.
- [CrossRef] [Google Scholar]
- Literature-based immunization recommendations for patients requiring immunosuppressive medications for autoimmune bullous dermatoses. Int J Dermatol. 2016;55:599-607.
- [CrossRef] [Google Scholar]
- A randomized double-blind trial of intravenous immunoglobulin for pemphigus. J Am Acad Dermatol. 2009;60:595-603.
- [CrossRef] [Google Scholar]
- High-dose intravenous immunoglobulin (IVIG) therapy in autoimmune skin blistering diseases. Clin Rev Allergy Immunol. 2010;38:186-95.
- [CrossRef] [Google Scholar]
- High-Dose Intravenous Immunoglobulin in Skin Autoimmune Disease. Front Immunol. 2019;10:1090.
- [CrossRef] [Google Scholar]
- First line treatment of pemphigus vulgaris with a novel protocol in patients with contraindications to systemic corticosteroids and immunosuppressive agents: Preliminary retrospective study with a seven year follow-up. Int Immunopharmacol. 2016;34:25-31.
- [CrossRef] [Google Scholar]
- Subcutaneous rituximab for the treatment of B-cell hematologic malignancies: A review of the scientific rationale and clinical development. Adv Ther. 2017;34:2210-31.
- [CrossRef] [Google Scholar]
- Intralesional rituximab in the treatment of refractory oral pemphigus vulgaris. JAMA Dermatol. 2015;151:878-82.
- [CrossRef] [Google Scholar]
- Sirolimus for acute pemphigus vulgaris: A case report and discussion of dualistic action providing for both immunosuppression and keratinocyte protection. J Am Acad Dermatol. 2011;65:684-6.
- [CrossRef] [Google Scholar]






