Translate this page into:
Clinical and electrophysiological characteristics of neuropathic pain in leprosy patients: A prospective cross-sectional study
Corresponding author: Prof. Gustavo Celeira de Sousa, Bernal do Couto Ave., 1003, Belém, Pará, 66055-080, Brazil. gustavo.c.sousa0@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Somensi DN, de Sousa EJ, Lopes G, de Sousa GC, Xavier MB. Clinical and electrophysiological characteristics of neuropathic pain in leprosy patients: A prospective cross-sectional study. Indian J Dermatol Venereol Leprol 2022;88:641-4.
Abstract
Introduction
Neuropathic pain is a common and disabling late complication of leprosy. We investigated the clinical and electrophysiological characteristics of neuropathic pain in leprosy patients by evaluating nerve conduction, sympathetic skin response (SSR) and A-waves.
Methods
Twenty one leprosy patients with neuropathic pain validated by the Douleur Neuropathique en 4 (DN4)Questionnaire were selected for study. Pain intensity was measured by the visual analog scale. Demographic and clinical data were collected for all patients. Clinical data included appraisal of the median, ulnar, radial, tibial and common peroneal nerves, assessment of the sympathetic skin response and conventional electrophysiological recordings.
Results
Among all electroneuromyographic presentations, multifocal mononeuropathy was still the most prevalent. Sensory loss was observed more frequently than motor deficits. As most patients presented advanced clinical forms of leprosy and were under treatment, this high mean was found and the ulnar nerve was most frequently affected. The sympathetic skin response was absent in 16 patients. Higher DN4 Questionnaire scores were observed in women and in those receiving corticosteroid therapy. These inferences are possible to be made, but our study's limitations don't allow us to be certain about it. The statistical significance found only permits us to evidence what we related on the textual part of the study.
Limitations
The small number of patients studied, the lack of sophisticated diagnostic methods for leprosy, as well as the difficulties in assessing nerve conduction were the main limitations of this study.
Conclusion
The neurophysiological and clinical findings in leprous neuropathy were modest despite the conspicuous neuropathic pain. Although electrophysiological studies are a vital tool to verify nerve damage, variations in the clinical presentation of leprosy neuropathic pain render the diagnosis challenging. Further studies are needed to describe the neurophysiological evolution of this disease.
Keywords
Electroneuromyography
electrophysiology
leprosy
neuropathic pain
neuropathy
Introduction
Leprosy is a common, treatable cause of peripheral neuropathy in many tropical and subtropical countries.1,2 Mycobacterium leprae has a distinct predilection for areas of low body temperature and a unique tropism for nerves. It frequently afflicts the ulnar nerve possibly due to its superficial location. The frequent delays in the diagnosis of leprosy allows the progression of nerve damage, often resulting in deformity.1,3-5
Neuropathic pain is a common symptom afflicting over 60% of patients with leprosy and may present before, during, or after treatment. It is frequently reported during leprosy reactions.6 The International Association for the Study of Pain defines neuropathic pain as “pain initiated or caused by a primary lesion or dysfunction in the nervous system.”6-9 Neuropathic pain originating in the peripheral nervous system as occurs in leprosy is characterized by injury to thin and nonmyelinated fibers. This type of injury is difficult to assess by conventional neurophysiological techniques such as electroneuromyography, although late latency evaluations such as the F-wave and sympathetic skin response are useful in their detection.1 Even in the absence of distal alterations, the A-wave tends to appear during the F-wave study in patients complaining of neuropathic pain.1,10,11
The previous studies have suggested that neuronal dysfunction occurs long before the clinical deterioration and the involvement of the nerve trunks.12-15 We conducted a systematic electrophysiological evaluation of leprosy patients with neuropathic pain, to correlate the clinical and electrophysiological findings.
Methods
Study design
We enrolled 21 leprosy patients with neuropathic pain from January to November 2014 from two reference centers for monitoring leprosy in Belém (Pará, Brazil): Centro Saúde Escola do Marco and Núcleo de Medicina Tropical. The study was approved in Universidade do Estado do Pará’s (UEPA) Ethics Committee on Human Research (CAAE) (Document No: 34662414.0.0000.5174).
Inclusion and exclusion criteria
Leprosy patients with neuropathic pain defined by the evaluation of the DN4 questionnaire were selected for the study. All patients were over 18 years of age and gave written consent. Patients at risk for other causes of neuropathy such as diabetes, alcoholism, HIV infection, or a family history of hereditary neuropathy were excluded from the study. The presence of acid-fast bacilli was not a criterion due to its low sensitivity for the diagnosis of leprosy.
Clinical evaluation
The diagnosis of leprosy in all study participants was made by a physician after clinical evaluation and examination of slit skin smears from both ear lobules for AFB. All patients were categorized based on the main clinical presentation as paucibacillary or multibacillary.14 The neuropathic pain was evaluated by a neurologist using both clinical criteria and the DN4 Questionnaire (score >4).8 All patients were then subjected to a clinical and electrophysiological evaluation.
Patients were classified as having neuropathic pain based on the grading system elaborated by Treede et al.8 A simplified neurological assessment was then conducted by a physiotherapist using Semmes-Weinstein monofilaments and a manual muscle-strength assessment was performed. The disability grade was evaluated according to Ministério da Saúde’s guidelines.16-18
Neurophysiological evaluation
The neurophysiological assessment was performed with a Neuropack MEB-9200, 4-channel EP/electroneuromyography system (Nihon Kohden, Tokyo, Japan) using the protocol detailed by Garbino et al.1 The sympathetic skin response was recorded with the low-frequency filter at 0.5 Hz, the high-frequency filter at 5000 Hz and sweep set at 5s. A single stimulus of 10–15 mA of 0.2 ms duration was delivered with a cathode placed proximally on the median and posterior tibial nerves. The recording electrodes were placed on both hands and feet (palmar, plantar as well as dorsal surfaces of hands and feet). Non-recordable responses were noted as abnormal.
Statistical analysis
The sample size was influenced by the difficulty in recruiting patients. The data were structured in a Microsoft Excel 2013 database and a BioEstat 5.0 program (Instituto Mamirauá, Tefé, Manaus, Brazil). The GraphPad Prism (GraphPad Software, San Diego, CA) was used for the generation of statistical results. The association of variables was assessed using the mean, median and standard deviation values, followed by the t-test and/or Pearson’s linear correlation. Confidence intervals (95%) and significance levels (α = 5%; P ≤ 0.05) were established.
Results
Mononeuropathy or multiple mononeuropathy patterns were most frequently seen [Table 1]. The A-wave was infrequent (5/21) in the nerve conduction studies (19%). There was significant difference between between those with mononeuropathy/ multiple mononeuropathy and the others (Chi-square, P = 0.0088). The sympathetic skin response was absent in 16 patients – on the soles in ten patients, on the palm in one patient and on both palms and soles in five patients [Figure 1]. The superficial fibular nerve was the most affected, followed by the sural nerve. In our study, some patients with thickened nerves had normal nerve conduction studies.
| Electroneuromyography patterns | n(%) |
|---|---|
| Mononeuropathy/multiple mononeuropathy | 5 (23.8) |
| Asymmetric sensorimotor neuropathy with focal slowing | 4 (19.0) |
| Asymmetric sensory neuropathy | 2 (9.5) |
| Asymmetric axonal sensorimotor neuropathy | 1 (4.8) |
| Symmetrical axonal sensorimotor neuropathy | 1 (4.8) |
| Others | 4 (19.0) |
| Normal | 4 (19.0) |
| Total | 21 (100.0) |
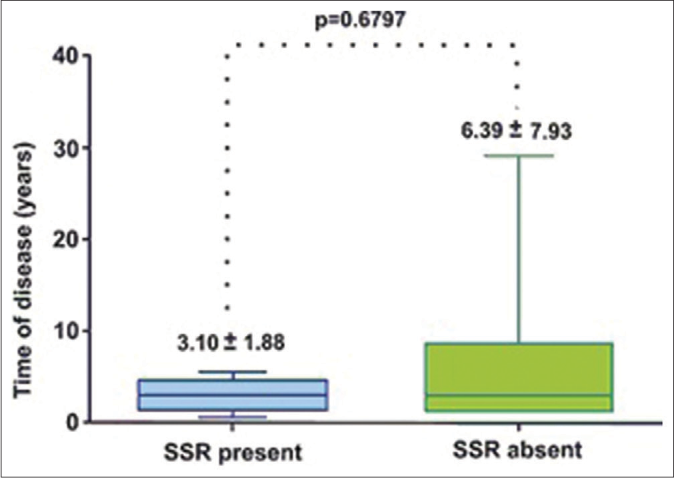
- Relationship between disease duration and sympathetic skin response of the 21 patients in the study
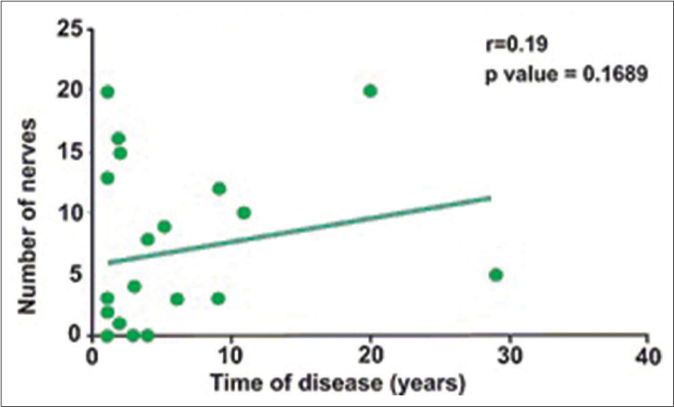
- Pearson correlation coefficient of affected nerves according to electroneuromyography and disease duration of the studied sample. Tropical Medicine Center, HCSM-UEPA—Belém-PA, 2014
Of the 21 enrolled patients, 13 were men and eight were women. The average age of the patients was 41.3 ± 12.4 years [Figure 1]. The majority of enrolled patients had multibacillary disease (19/21 patients). Seventeen of the patients had dimorphic leprosy and 11 patients had type I reaction [Table 2]. The duration of disease at presentation ranged from 13 to 72 months in 12 patients and the duration of multidrug therapy ranged from 7 to 12 months in 13 patients [Figure 1]. Seventeen of the 21 patients were on corticosteroid therapy [Tables 3 and 4]. The ulnar nerve was most frequently affected and 18 patients exhibited some degree of disability [Table 2].
| Clinical characteristics | EMG, n(%) | t TEST | |
|---|---|---|---|
| Changed | Normal | DN4 | |
| P-value | |||
| Clinical form | |||
| Mutibacillar | 16 (94.1) | 3 (75) | 0.8271 |
| Paucibacillar | 1 (5.9) | 1 (25) | |
| Leprosy reaction | |||
| With reaction | 9 (52.9) | 2 (50) | 0.6507 |
| Without reaction | 8 (47.1) | 2 (50) | |
| Grade of disability | |||
| Grade 0/I | 8 (47.1) | 4 (100) | 0.1472 |
| Grade II | 9 (52.9) | 0 (0) | |
EMG: Electroneuromyography
| Variables | Mean | Median | SD | VAS P-value |
|---|---|---|---|---|
| Sex | ||||
| Female | 8.38 | 9 | 0.88 | 0.0359* |
| Male | 7.17 | 7 | 1.27 | |
| Age | ||||
| <45 | 7.5 | 8 | 1.2 | 0.82 |
| >45 | 7.9 | 7 | 1.1 | |
| Corticosteroid | ||||
| Yes | 8 | 8 | 0.9 | 0.0009* |
| No | 6 | 6 | 0.7 | |
| Sympathetic skin response | ||||
| Present | 8 | 7.8 | 1.2 | 0.7726 |
| Absent | 7.5 | 7.6 | 1.2 | |
| Reaction | ||||
| Yes | 8.1 | 8 | 1.22 | 0.1004 |
| No | 7.2 | 7 | 1.13 | |
| Grade of disability | ||||
| Grade 0/I | 7.7 | 8 | 1.2 | 0.1593 |
| Grade II | 7.7 | 8 | 1.2 |
| Variables | Mean | Median | SD | P-value |
|---|---|---|---|---|
| Sex | ||||
| Female | 8 | 9 | 1.7 | 0.0822 |
| Male | 6.8 | 7 | 1.3 | |
| Age | ||||
| <45 | 7.1 | 7.5 | 1.5 | 0.8914 |
| >45 | 7.3 | 7.5 | 1.5 | |
| Corticosteroids | ||||
| Yes | 7.5 | 7.5 | 1.5 | 0.2873 |
| No | 6.3 | 6.5 | 1.8 | |
| SSD | ||||
| Present | 8 | 9 | 1.5 | 0.164 |
| Absent | 7.1 | 7 | 1.5 | |
| Reaction | ||||
| Yes | 7.7 | 8 | 1.4 | 0.2453 |
| No | 5 | 7.5 | 1.6 | |
| Grade of disability | ||||
| Grade 0/I | 7.3 | 7.5 | 1.8 | 0.2008 |
| Grade II | 6.7 | 7.5 | 2 |
Discussion
Neuropathic pain is a frequently overlooked symptom in the management of leprosy. Patients during or after multidrug therapy have a high prevalence of neuropathic pain.10,19-22 Although the frequency and nature of the neuropathic pain in leprosy is unclear, it seems that late diagnosis of leprosy and the evolution of nerve damage contributes to the onset of pain [Table 3] Neuropathic pain is most commonly seen in borderline and MB cases.10,23-26
Multifocal mononeuropathies are the most prevalent neurophysiological pattern and the ulnar nerve is most often affected.27,28 Electromyography is an excellent tool for early detection and monitoring nerve damage.2,29-31 In our study, some patients with thickened nerves had normal nerve conduction studies. This clinicoelectrophysiological dissociation may be due to the involvement of few fascicles initially sparing of the faster conducting fibers.32-34 Contrary to the findings of Garbino et al., we found a low prevalence of A-waves which may be due the fact that most patients had been treated or were under treatment with corticosteroids [Table 4].35-36
Limitations
Neuropathic pain is a subjective symptom and it was difficult to characterize and evaluate. Many patients refused consent when the electromyography procedure was explained which may result in bias. Patients often arrived late in the course of the disease, or with unknown comorbidities causing pain of nociceptive nature. Thus, further studies are needed to validate the findings of this study and overcome these limitations.
Conclusion
In this study, mononeuropathy and multiple mononeuropathy were the most common patterns and the ulnar nerve was the most affected nerve trunk. The neurophysiological assessment did not reveal the predominance of any specific electromyographic pattern and some patients in this study showed normal nerve conduction studies. The DN4 questionnaire scale revealed more intense pain in female patients and in patients on corticosteroids.
Acknowledgments
This study was conducted independently of funding agencies and pharmaceutical companies. We are grateful to Geraldo Macedo, MD for his helpful contribution to statistical analyses.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Association between neuropathic pain and A-waves in leprosy patients with Type 1 and 2 reactions. J Clin Neurophysiol. 2011;28:329-32.
- [CrossRef] [PubMed] [Google Scholar]
- Investigation of neuropathic pain in treated leprosy patients in Ethiopia: A cross-sectional study. Pain. 2012;153:1620-4.
- [CrossRef] [PubMed] [Google Scholar]
- Contact-dependent demyelination by Mycobacterium leprae in the absence of immune cells. Science. 2002;296:927-31.
- [CrossRef] [PubMed] [Google Scholar]
- Leprosy and the peripheral nervous system: Basic and clinical aspects. Muscle Nerve. 2004;30:393-409.
- [CrossRef] [PubMed] [Google Scholar]
- Pain in leprosy: General challenges of a singular disease. Pain. 2015;156:983-5.
- [CrossRef] [PubMed] [Google Scholar]
- What do general neurologists need to know about neuropathic pain? Arq Neuropsiquiatr. 2009;67:741-9.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630-5.
- [CrossRef] [PubMed] [Google Scholar]
- Newer management options in leprosy. Indian J Dermatol. 2013;58:6-11.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropathic pain in leprosy patients. Int J Lepr Other Mycobact Dis. 2004;72:134-8.
- [CrossRef] [Google Scholar]
- Diagnosis and impact of neuropathic pain in leprosy patients in Nepal after completion of multidrug therapy. PLoS Negl Trop Dis. 2018;12:e0006610.
- [CrossRef] [PubMed] [Google Scholar]
- Ulnar nerve sonography in leprosy neuropathy. J Med Ultrason (2001). 2016;43:137-40.
- [CrossRef] [PubMed] [Google Scholar]
- Secretaria de Vigilância em Saúde, Portaria No. 3125 de 7 de Outubro de 2010 Brasília: Ministério da Saúde; 2010.
- [Google Scholar]
- Clinical, electro-physiological, and immunopathological study of peripheral nerves in Hansen's disease. Lepr Rev. 2001;72:35-49.
- [CrossRef] [PubMed] [Google Scholar]
- Degree of disability, pain levels, muscle strength, and electromyographic function in patients with Hansen's disease with common peroneal nerve damage. Rev Soc Bras Med Trop. 2012;45:375-9.
- [CrossRef] [PubMed] [Google Scholar]
- Avaliação dos membros superiores para a prevenção de incapacidades In: Opromolla VA, Baccarelli R, eds. Prevenção de Incapacidades e Reabilitação em Hanseníase. Bauru: Instituto Lauro de Souza Lima; 2003. p. :72-81.
- [Google Scholar]
- Secretaria de Vigilância em Saúde, Manual de Prevenção de Incapacidades (3rd ed). Brasília: Ministério da Saúde; 2008.
- [Google Scholar]
- Using screening tools to identify neuropathic pain. Pain. 2007;127:199-203.
- [CrossRef] [PubMed] [Google Scholar]
- Translation to Portuguese and validation of the Douleur Neuropathique 4 questionnaire. J Pain. 2010;11:484-90.
- [CrossRef] [PubMed] [Google Scholar]
- High prevalence of vasomotor reflex impairment in newly diagnosed leprosy patients. Eur J Clin Invest. 2005;35:658-65.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropathic pain and psychological morbidity in patients with treated leprosy: A cross-sectional prevalence study in Mumbai. PLoS Negl Trop Dis. 2011;5:e981.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and characteristics of neuropathic pain in the people affected by leprosy in China. Lepr Rev. 2012;83:195-201.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and characteristics of neuropathic pain in leprosy patients treated years ago. Pathog Glob Health. 2014;108:186-90.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropathic pain in people treated for multibacillary leprosy more than ten years previously. Lepr Rev. 2008;79:270-6.
- [CrossRef] [PubMed] [Google Scholar]
- Psychological distress and quality of life in leprosy patients with neuropathic pain. Lepr Rev. 2014;85:186-93.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropatia Hanseniana: Aspectos fisiopatológicos, clínicos, dano neural e regeneração In: Opromolla DV, ed. Noções de Hansenologia. Bauru: Centro de Estudos Dr. Reynaldo Quagliato; 2000. p. :79-89.
- [Google Scholar]
- Leprosy neuropathy: Clinical presentations. Arq Neuropsiquiatr. 2013;71:661-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and electrophysiological approach to periferic neuropathies. Acta Fisiátrica. 1998;5:11-7.
- [Google Scholar]
- The clinical and neurophysiological study of leprosy. Pak J Med Sci. 2014;30:501-6.
- [Google Scholar]
- Comprehensive electrophysiology in leprous neuropathy-is there a clinicoelectrophysiological dissociation? Clin Neurophysiol. 2016;127:2747-55.
- [CrossRef] [PubMed] [Google Scholar]
- The INFIR cohort study: Assessment of sensory and motor neuropathy in leprosy at baseline. Lepr Rev. 2005;76:277-95.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnóstico e tratamento da neuropatia, Projeto Diretrizes e Bases In: Associação Médica Brasileira e Conselho Federal de Medicina. 2003.
- [Google Scholar]
- Eletroneuromiografia em hanseníase In: Cirurgia Reparadora e Reabilitação em Hanseníase. Duerksen and Virmond. Bauru: ALM International; 1997. p. :93-104.
- [Google Scholar]
- Chronic pain in leprosy: New aspects to be considered. Pain Manag. 2013;3:201-10.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathological and clinical findings in leprosy patients with chronic neuropathic pain: A study from Hyderabad, India. Lepr Rev. 2007;78:369-80.
- [CrossRef] [PubMed] [Google Scholar]






