Translate this page into:
Brachial plexopathy with ulnar nerve abscess in leprosy: A case showing importance of magnetic resonance neurography with clinical, imaging and histopathological correlation
Corresponding author: Dr. Ashima Mittal, Senior Resident, Radiodiagnosis, Dayanand Medical College & Hospital, Room number- N-48, PG Hostel, Ludhiana, Punjab, India. ashima.mittal44@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mittal A, Singh Dhanota DP, Saggar K, Ahluwalia A. Brachial plexopathy with ulnar nerve abscess in leprosy: A case showing importance of magnetic resonance neurography with clinical, imaging and histopathological correlation. Indian J Dermatol Venereol Leprol 2022;88:385-8.
Sir,
Leprosy is the most common preventable neuropathy in endemic areas, caused by Mycobacterium leprae. Due to its unique tropism for the nerves, patients with all forms of leprosy, but particularly those with the borderline tuberculoid form may develop abscesses of nerves, most commonly the ulnar nerve. There is a relative paucity of literature on brachial plexus involvement in leprosy and its magnetic resonance neurography appearance, which is limited to only a few studies and case reports. This case report illustrates the involvement of brachial plexus in addition to ulnar nerve in a patient with leprosy and the indispensable role of magnetic resonance neurography in early detection of brachial plexus neuritis.
A 24-year-old male was referred to department of radiology in our hospital for evaluation of progressive left ulnar palsy with sensorimotor symptoms. He complained of swelling and pain around inner aspect of left elbow joint for one year, along with progressive hand weakness and occasional numbness. On physical examination, left ulnar nerve was markedly thickened and tender, extending proximally till the tender swelling on the medial aspect of left upper arm, which was soft and fluctuant with a size of 6 × 4 cm, suggestive of ulnar nerve abscess [Figure 1a]. Motor examination of left hand revealed partial claw hand deformity and the patient was unable to flex metacarpophalangeal joints and extend interphalangeal joints of fourth and fifth fingers of left hand [Figure 1b]. There was flattening of hypothenar eminence, guttering of interosseous spaces Figure 1b along with failure of adduction and abduction of fourth and fifth fingers of left hand. Sensory examination revealed near-complete absence of touch, pain, and temperature sensations over the ulnar distribution of left hand. There was no thickening or tenderness of any other peripheral nerve. Cutaneous examination revealed a single, oval reddish-brown plaque of size 4 × 3 centimetres which was mostly well-defined except at its upper edge, seen on left upper cheek in pre-auricular location [Figure 1c]. Sensations were intact over the plaque and there was no cutaneous nerve twig along its border. The ocular and other mucosal examination was normal. Corneal sensations were preserved.
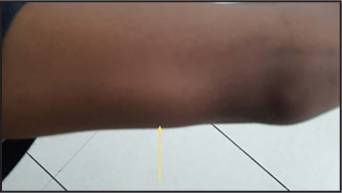
- Swelling on medial aspect of left upper arm, a few centimetres proximal to elbow joint (yellow arrow)
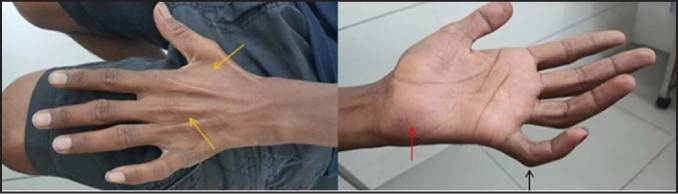
- The left-hand shows guttering of interosseous spaces (yellow arrows), flattening of hypothenar eminence (red arrow), partial claw deformity of fourth and fifth fingers (black arrow)

- A single, well-defined oval reddish-brown plaque of size 4 × 3 centimetres on left side of face (yellow arrow)
High-resolution ultrasonography using a linear probe at 12 MHz (Logiq E9 R2.0 XD Clear, GE Healthcare) revealed diffuse heterogeneous hypoechoic thickening of left ulnar nerve involving cubital tunnel area and region proximal to tunnel for a length of approximately 10 to 11 cm with loss of normal fascicular architecture [Figure 2A and B]. The maximum cross-sectional diameter of the nerve was 10 mm. Colour doppler scan showed intraneural hypervascularity [Figure 2C and D]. Also, a thick-walled collection/abscess measuring 2 × 1.2 cm with internal echoes and liquefied component was seen in close approximation and communicating with the thickened nerve, a few centimetres proximal to elbow joint, showing peripheral vascularity [Figure 2E and F]. The ultrasonography was also done for other peripheral nerves of both upper limbs and did not reveal any abnormality.

- (A) Axial and (B) longitudinal USG images showing hypoechoic thickening of the left ulnar nerve (marked with calipers). An eccentric intrasubstance anechoic area is noted suggestive of abscess formation (white arrow). (C) Axial and (D) longitudinal colour doppler images showing increased intraneural vascularity. (E) Axial USG image showing a thick-walled collection (marked with calipers) with internal liquefied component communicating with the thickened nerve (black arrows). (F) On colour doppler, the abscess shows peripheral vascularity
Magnetic resonance neurography (3T Magnetom Skyra MRI scanner) was performed subsequently to evaluate the proximal extent of nerve involvement using routine MR sequences including axial T1, axial T2, axial and coronal proton density fat saturated, and in addition using coronal and sagittal 3D short tau inversion recovery sequence, 3D diffusion-weighted imaging/apparent diffusion coefficient, axial T1 FS pre- and post-contrast sequences. It revealed diffuse hyperintense thickening of C8-T1 roots, inferior trunk, divisions and medial cord of brachial plexus Figure 3a, extending distally to involve left ulnar nerve in upper arm till the level of cubital tunnel with loss of fascicular architecture [Figure 3b, A-B]. The maximum thickness of the nerve was 8.8 mm at distal aspect of humerus. The nerve fascicles appeared swollen and surrounded by thickened hypointense perineurium and epineurium. Post-contrast, it showed thick irregular peripheral enhancement with significant diffusion restriction on diffusion-weighted/apparent diffusion coefficient images [Figure 3b, C-D]. There was surrounding soft tissue oedema. A proton density fat saturated hyperintense collection measuring 2.2 × 1.4 cm was seen closely abutting and communicating with the thickened nerve at distal aspect of humerus, approximately 6 cm proximal to elbow joint [Figure 3b, E-F]. Post-contrast, it showed thick peripheral enhancement with significant diffusion restriction on diffusion-weighted/apparent diffusion coefficient images [Figure 3b, G-H].

- MIP (Maximum intensity projection) reconstructed MR image from coronal 3D STIR shows diffuse hyperintense thickening of C8 and T1 roots (yellow arrows), inferior trunk (red arrow), divisions (white arrow) and medial cord (green arrow) of brachial plexus, extending to involve ulnar nerve (blue arrow) on left side

- (A) Axial and (B) coronal PD FS MR images show hyperintense thickening of the left ulnar nerve at level of cubital tunnel (white arrows). (C) Axial post-contrast image shows thick peripheral enhancement of the thickened nerve (white arrow). (D) Axial DWI and ADC images show significant diffusion restriction in the thickened nerve (white arrows). (E) Axial and (F) coronal PD FS images show a hyperintense collection (yellow arrows) communicating with the thickened nerve (white arrows) at distal aspect of humerus. (G) Axial post-contrast image shows thick peripheral enhancement of the collection (yellow arrow) as well as of the nerve (white arrow). (H) Axial DWI and ADC images show marked diffusion restriction in the collection (yellow arrows)
Based on these imaging findings of brachial plexopathy with ulnar neuritis and abscess formation, a diagnosis of infective pathology likely to be leprosy was made.
Consequently, ultrasonography-guided fine-needle aspiration from the collection yielded yellowish pus and the pus smear was negative for acid fast bacilli (AFB). Slit skin smear from ear lobule and the plaque on the cheek was negative. A skin biopsy from the plaque showed presence of epithelioid cell granulomas in the dermis with lymphocytic collar and absence of caseous necrosis. The granulomas were not hugging the overlying epidermis [Figure 3c]. Ziehl-Neelsen stain for AFB was negative. With these findings, a diagnosis of borderline tuberculoid leprosy with ulnar nerve abscess and Grade 2 deformity of left hand was made.

- Hematoxylin and eosin stained section of plaque on left cheek showing presence of dermal epithelioid cell granulomas with lymphocytic collar and no evidence of caseous necrosis. (H and E, 100×)
The patient was started on WHO multibacillary multi-drug therapy (MB-MDT) constituting rifampin (600 mg/month), clofazimine (300 mg/month and 50 mg daily) and dapsone (100 mg daily) for 12 months, and prednisolone 0.5 mg/kg for management of nerve abscess and ulnar neuritis. Corticosteroids were gradually tapered and stopped after six months. The patient has completed 10 months of MB-MDT and is on regular monthly follow-up. The swelling of nerve abscess and accompanying neuritis were observed to reduce significantly, thus conservative management was continued. No recurrence has been observed after stopping steroids. However, there was no improvement in sensory and motor deficits. The patient was advised supportive and rehabilitative physiotherapy and also advised protection from extremes of temperature and injury.
In India, a nerve abscess develops in approximately 1.3% of patients with leprosy. Young children and teenagers account for the majority of cases.1 Although any peripheral nerve may be enlarged, those most commonly affected are ulnar, posterior auricular, peroneal and posterior tibial nerves. If patients with affected nerves are not treated promptly with glucocorticoids, irreversible nerve damage may result in as little as 24 hours.2 Imaging methods such as ultrasonography and MRI can be used for early detection of nerve damage in leprosy.3 Biopsy from skin lesions is important for confirming the diagnosis. Nerve biopsy is warranted in cases lacking typical cutaneous manifestations.4
Brachial plexus involvement in leprosy and its magnetic resonance neurography appearance has been rarely described in the literature.5 Magnetic resonance neurography is indispensable in the evaluation of brachial plexus and peripheral nerves. It allows fine detailed evaluation of nerve anatomy and pathology, internal architecture, course, caliber and involvement of regional muscles. A typical magnetic resonance neurography protocol includes high-resolution 2D and 3D sequences, i.e., axial T1, axial T2, axial and coronal proton density fat saturated, coronal and sagittal 3D short tau inversion recovery sequence, 3D diffusion-weighted imaging, axial T1 FS pre- and post-contrast. On MRI, thickening of the plexus is seen as T2/short tau inversion recovery sequence hyperintensity which may be symmetric or asymmetric.
Isolated involvement of the brachial plexus in leprosy has been reported earlier in only a few case series. Awareness of MRI findings in leprosy including brachial plexus involvement becomes more important in the current scenario wherein the pure neuritic form of leprosy is frequently encountered, helping in early detection, effective management and prevention of nerve damage.5
It has been observed that brachial plexus involvement is commonly seen in the borderline tuberculoid variant of leprosy.5 This may be because of the fact that cases towards the tuberculoid end of the spectrum mount a better cell-mediated immune response leading to the formation of epithelioid granulomas and perineural fibrosis manifested on imaging as nerve thickening. Thus, long term management of brachial plexopathy due to leprosy, in this case, will depend on the inflammatory process triggered by Mycobacterium leprae.
Specific modifications in treatment in cases of leprosy with brachial plexopathy have been described. Therapy includes non-steroidal anti-inflammatory drugs, glucocorticoids, calcitonin and bisphosphonates, tricyclic antidepressants, membrane stabilizers like carbamazepine, gabapentin, lidocaine and mexiletine, N-methyl-D-aspartate receptor antagonists and ketamine.6 Topical capsaicin cream, dimethyl sulfoxide and lignocaine cream can also be used.
Physical and supportive measures consist of exercise therapy, occupational therapy and transcutaneous electrical nerve stimulation. Regional anaesthetic techniques like sympathetic ganglion block with local anaesthetics, serial conduction blocks of brachial plexus and neuromodulation techniques like spinal cord stimulation have been tried with some success. The use of various treatments will depend upon symptomatology or severity of the case. The goal of treatment is to restore function with a multi-disciplinary approach.
There are varied opinions regarding the duration of steroids to be used in patients of leprosy with brachial plexopathy. Cochrane review7 published in 2008 to assess the effectiveness of corticosteroid in treating nerve damage in leprosy concluded that a five-month steroid regime was significantly more beneficial than a three-month regime. However, Shetty et al.8 in 2010 studied the effect of corticosteroids on nerve damage in leprosy and concluded, on the basis of nerve conduction studies, that MDT along with steroids was not very efficacious in the prevention or reversal of nerve damage. They also concluded that a better immunomodulatory drug or a modified corticosteroid regime is needed.
Leprosy is an important differential diagnosis to be considered in cases of brachial plexopathy since it is the most common preventable neuropathy in endemic areas. In our case report with brachial plexitis and ulnar neuritis, presence of a skin lesion and its histopathological examination clinched the diagnosis of borderline tuberculoid leprosy. However, in pure neuritic cases, a nerve biopsy may be needed along with the imaging studies. Post-treatment imaging will help us in assessing treatment outcome including any neurological sequelae. However, in our patient, high-resolution ultrasonography and magnetic resonance neurography were not repeated after treatment initiation because of financial constraints. Further studies are needed to know about the importance of post-treatment magnetic resonance neurography in these patients.
We are reporting a unique and rare case of brachial plexus neuritis with ulnar nerve abscess in leprosy, highlighting the significant role of MR neurography in the evaluation of brachial plexus and peripheral nerves, aiding in early diagnosis of leprosy and effective patient management, thus preventing nerve damage and subsequent disabilities.
Acknowledgement
We immensely acknowledge the help provided by Dr. Sukhjot Kaur, Associate Professor, Department of Dermatology, Dayanand Medical College and Hospital, Ludhiana, Punjab in manuscript preparation and editing, and providing valuable information about the clinical aspects and management of the patient.
Declaration of patient consent
Patient’s consent is not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Ultrasonography and magnetic resonance imaging of ulnar nerve abscess in leprosy. Med J Armed Forces India. 2016;72:78-81.
- [CrossRef] [PubMed] [Google Scholar]
- Leprosy In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison's Principles of Internal Medicine (20th ed). New York: McGraw-Hill; 2018. p. :1259-1266.
- [Google Scholar]
- Magnetic resonance imaging of ulnar nerve abscess in leprosy: a case report. Lepr Rev. 2006;77:381-5.
- [Google Scholar]
- US and MR imaging of peripheral nerves in leprosy. Skeletal Radiol. 2000;29:142-50.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroimaging in leprosy: the nerves and beyond. Radiol Infect Dis. 2020;7:12-21.
- [CrossRef] [Google Scholar]
- Complex regional pain syndrome secondary to leprosy. Pain Medicine. 2012;13:1067-71.
- [CrossRef] [PubMed] [Google Scholar]
- Corticosteroids for treating nerve damage in leprosy. A Cochrane review. Lepr Rev. 2008;79:361-71.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of corticosteroids usage on bacterial killing, clearance and nerve damage in leprosy; part 3-8. Study of two comparable groups of 100 multibacillary (MB) patients each, treated with MDT + steroids vs. MDT alone, assessed at 6 months post-release from 12 months MDT. Lepr Rev. 2010;81:41-58.
- [CrossRef] [PubMed] [Google Scholar]





