Translate this page into:
Evaluation of the safety and efficacy of platelet-rich plasma in the treatment of female patients with chronic telogen effluvium: A randomised, controlled, double-blind, pilot clinical trial
Corresponding author: Dr Amr Abdelhamed, Department of Dermatology, Venereology and Andrology, Faculty of Medicine, Sohag University, Sohag, Egypt. amr_abdelhamed@med.sohag.edu.eg
-
Received: ,
Accepted: ,
How to cite this article: Ezz El-Dawla R, Abdelhaleem M, Abdelhamed A. Evaluation of the safety and efficacy of platelet-rich plasma in the treatment of female patients with chronic telogen effluvium: A randomised, controlled, double-blind, pilot clinical trial. Indian J Dermatol Venereol Leprol 2023;89:195-203.
Abstract
Background:
Chronic telogen effluvium is characterised by diffuse loss of hair of the scalp. One of the emerging lines of treatment is platelet-rich plasma. However, not much of published data exist.
Aims:
A pilot study was conducted on chronic telogen effluvium patients to evaluate the efficacy and safety of platelet-rich plasma, and to compare two different methods of platelet-rich plasma preparation.
Methods:
The study included 30 female patients with chronic telogen effluvium. Patients were randomised into three groups: Group (1): Special platelet-rich plasma tubes centrifuged at 3500 rpm; Group (2): Ordinary laboratory tubes centrifuged at 1000 rpm; Group (3): Normal saline as a placebo. Patients' evaluation was done with visual analog scale, hair pull test, trichoscopy, photos, satisfaction questionnaire, and safety. All patients received four monthly sessions. Patients were evaluated one month and three months after the last session.
Results:
The hair pull test,visual analogue scale, and patient satisfaction results showed a statistically significant difference between group 1 vs. group 3 and group 2 vs.group 3 at one and three months after the sessions, while there was no difference between group1 vs. group 2. Trichoscopy results (baseline, one and three months after treatment) showed a significant increase in hair density and thickness in the frontal area, temporal area, and the vertex in groups 1 and 2 only. There was no statistically significant difference between the three groups with regards to side effects.
Limitations:
The sample size was small with ten patients in each group. Furthermore, the follow-up of patients was for only three months.
Conclusions:
Platelet-rich plasma could be considered as a promising therapy for patients with chronic telogen effluvium with an excellent safety profile. The ordinary laboratory low-cost tubes might be a reliable alternative to the expensive special platelet-rich plasma kits tubes. The trial registry number is PACTR202006539654415.
Keywords
Diffuse hair loss
hair density
platelet-rich plasma
telogen effluvium
trichoscopy
Plain Language Summary
Chronic telogen effluvium (CTE) is a common hair disorder characterized by diffuse hair falling. It is prevalent worldwide with no known prevalence as many cases are subclinical. Many lines of treatment are available for this problem with platelet-rich plasma (PRP) as an emerging treatment. PRP is prepared from the patients' blood and injected into the scalp. PRP provides hair with many growth factors. This study, from Egypt, aimed to evaluate the efficacy and safety of PRP in chronic telogen effluvium and compared between two methods of PRP preparation with one method producing higher platelet. Patients received monthly four sessions and followed up to three months after the end of treatment. The authors found that PRP showed a significant improvement in patients with chronic telogen effluvium at the clinical level and with regard to patient satisfaction. In a comparison between two different methods of PRP preparation, with one method capable of producing plasma with significantly higher platelet concentration, there was no significant difference in hair improvement. The authors suggested that a certain level of platelet concentration is needed for hair improvement. Beyond this level, more concentration is not associated with more clinical improvement.
Introduction
Chronic telogen effluvium is one of the most common causes of diffuse hair loss in females. It is characterised by diffuse hair loss of insidious onset lasting for more than six months. It is usually preceded by a triggering factor including physical or emotionally stressful events.1 In most cases of chronic telogen effluvium, no underlying cause could be identified; however, iron deficiency anemia is considered a common cause.2 Most chronic telogen effluvium patients complain of reduced scalp hair density. Scalp examination usually reveals normal hair thickness with a commonly positive hair pull test.3 The principle of treatment of chronic telogen effluvium is mainly patient reassurance,1 and treatment of the underlying cause, if identified.4 One of the emerging treatment modality is use of platelet-rich plasma.5
Platelet-rich plasma is a biological product obtained after centrifugation of autologous blood, resulting in a portion of the plasma that has a higher platelet concentration above the baseline. Platelet-rich plasma can be prepared by several methods; however, there is no standard protocol for platelet-rich plasma preparations.6 Platelet-rich plasma contains a wide range of growth factors, chemokines, cytokines, and many plasma proteins.7 These growth factors might act on the stem cells in the bulge region of the hair follicle leading to new hair development and facilitating neovascularisation.8
Platelet-rich plasma is widely used in various hair disorders especially androgenetic alopecia.5 There is a lack of well-designed studies evaluating the platelet-rich plasma efficacy in patients with chronic telogen effluvium. Therefore, this pilot trial was conducted to evaluate the efficacy and safety of platelet-rich plasma in chronic telogen effluvium using two different methods of preparations.
Material and Methods
A pilot study was conducted on 30 patients with chronic telogen effluvium who attended Sohag university hospital, Egypt; in the period from March 2018 to February 2020. Informed consent was obtained from all participants. The study was approved by the Research and Ethical Committee at Sohag Faculty of Medicine, Sohag University, Egypt. The trial registry number is PACTR202006539654415. Both the participants and the outcome assessors were blinded to the type of treatment. Insulin syringe that were used in injecting saline and PRP were covered with adhesive plaster. CONSORT flow chart of the study is shown in Figure 1.

- A CONSORT flow chart of the study
Inclusion criteria
The study included female patients aged between 18 and 55 years. Patients were allocated by simple randomization into three groups of ten patients each, as follows: Group (1): Platelet-rich plasma injections using special platelet-rich plasma tubes centrifuged at 3500 rpm; Group (2): Platelet-rich plasma injections using ordinary laboratory tubes centrifuged at 1000 rpm; and Group (3): Injection with normal saline solution as a placebo. Sample size determination was done according to the average number of participants of platelet-rich plasma clinical trial in androgenetic alopecia patients as there were no previous clinical trials on chronic telogen effluvium patients. A review of 12 clinical studies showed an average number of participants was 25 (range 10–64).9 Hence, twenty patients were included for platelet-rich plasma and ten patients for placebo.
Exclusion criteria
Patients having dermatological diseases affecting the scalp, thyroid dysfunction, chronic medical diseases, pregnant or lactating women, anemia (haemoglobin level <10mg/dl), thrombocytopenia (platelet count <100,000/µL), coagulopathies, patients on anticoagulant therapy such as aspirin, and patients receiving topical or systemic treatment for hair loss in the last three months before the study; were excluded from the study.
Methods
Initial evaluation
Medical history: It included personal history, duration of the disease, medical diseases, and previous hair treatment.
General examination: It was done to exclude any systemic diseases.
Laboratory investigations: Complete blood count (Miracell, made in Egypt), thyroid function tests, and serum ferritin level (Cobas E411, made in Germany)
-
Dermatological evaluation:
General dermatological evaluation: Local examination of the scalp and hair examination were done.
Photos: Photos were taken using a smartphone (iPhone 7).
Visual analog scale: It helps to measure hair shedding which was scored on a scale of onesix. Patients were asked to look at the scale and point to the photograph that best describes the amount of hair shed on an average day. Grades one-three were considered normal, grade four was borderline, while grades five and six indicated excessive shedding.10
Hair pull test: A bundle of hair was grasped between the thumb, index, and middle finger from the base near the scalp, and firmly and slowly pulled. The extracted hairs were counted. Normally only four to six hairs are pulled, but it is increased in chronic telogen effluvium patients.11
Trichoscopy: It was done to diagnose and evaluate chronic telogen effluvium which is usually diagnosed by exclusion. The trichoscope used was (Micro-viewer 1.3 MP, made in China), using (×200) magnification. Three trichoscopic photos were taken from each of the frontal, temporal areas, and vertex. The photos were taken during the follow-up from the same sites using a ruler and measuring the distance from the photo site and inner and outer eyebrows as a landmark for each patient. Trichoscopic photos were evaluated blindly by using digital computerized software (Compare view SW, by STR, CA-USA, by Dr. Steve John). The outcome measures of hair density and thickness represented the average of these three photos representing each site to ensure the validity of the trichoscopic results.
Methods of treatment
All patients received four sessions, one session every 4 weeks. All patients were blinded as regards the composition of intradermal injections. About two ml of platelet-rich plasma or saline was injected in each session. In all the groups, topical alcohol and anesthetic spray (xylocaine spray, AstraZeneca company) were used before the session.
Group (1): Platelet-rich plasma was prepared by special platelet-rich plasma kits tubes (Zoom kits, Unex Med company, made in Italy) that contain 1 ml sodium citrate 3.8%. ten ml of blood was drawn from the patient and placed in the tube which was centrifuged at 3500 rpm (2054 G) for ten minutes as recommended by the manufacturing company. The centrifuge used was (80-1 Electronic Centrifuge, made in China)
Group (2): Platelet-rich plasma was prepared by ordinary laboratory tubes (sterile plastic conical 15 cm tubes, made in Egypt). Sodium citrate, 1 ml, 3.8% (Bio-Tek Egypt Diagnostic company, made in Egypt) was placed in the tube. Ten ml of blood was drawn from each of the patients and placed in the tube which was centrifuged at 1000 rpm (167.7 G) for ten minutes as we found out after several trials to be having the best platelet concentration using the same centrifuge.
Group (3): The patients were injected with normal saline solution as a placebo.
Evaluation of treatment
The patients were evaluated one month and three months after the last session by the use of visual analog scale, hair pull test, and trichoscopy. Also, a five-item questionnaire form was asked to be filled by patients to evaluate their satisfaction with the treatment.12 This questionnaire, though developed and validated for evaluating androgenetic alopecia in males, was used by several researchers evaluating hair loss in females as well.13 Furthermore, safety was evaluated by monitoring any local or systemic side effects.
Statistical analysis
Statistical analysis was carried out using SPSS statistics software version 23. The variables that were not normally distributed; were described by the median (min-max). Qualitative data were expressed by numbers and percentages. For quantitative data, comparison between two groups was done with the Wilcoxon test or Mann-Whitney U test according to data normality, while comparison between 3 groups was done with the Kruskal-Wallis test. For qualitative data, the Chi-square test and Monte Carlo Exact test were used. For correlation tests, the Pearson or Spearman tests were used. The test was considered significant with P<0.05.
Results
The median age of patients was 31.5, 29, and 27 years in the group 1, 2, and 3, respectively. There was no statistically significant difference as regards age, duration of hair loss, and other laboratory characteristics between the three groups as shown in [Table 1]. Platelet concentration was significantly higher in group 1 (1670 × 10^9/l) versus group 2 (884.5 ×10^9/l) (P=0.001) as shown in [Figure 2]. In group 1, the platelets increased (from 4.04 to 7.8) fold with median (6.5), while in group 2, the platelets increased (from 2.55 to 3.14) fold with median (2.88).
| Group (1) (n=10) | Group (2) (n=10) | Group (3) (n=10) | Test of significance (P) | |
|---|---|---|---|---|
| Median (Min-Max)/No (%) | Median (Min-Max)/No (%) | Median (Min-Max)/No (%) | ||
| Age (years) | 31.5 (24–45) | 29 (19–49) | 27 (18–44) | †P=0.18 |
| Marital status | ‡P=0.17 | |||
| Single | 2 (20) | 60 (60) | 5 (50) | |
| Married | 8 (80) | 40 (40) | 5 (50) | |
| Occupation | §P=0.14 | |||
| Housewife | 5 (50) | 3 (30) | 5 (50) | |
| Employee | 5 (50) | 0 | 0 | |
| Student | 0 | 7 (70) | 5 (50) | |
| Duration of hair loss (years) | 3.5 (1–5) | 2 (1–5) | 2 (1–5) | †P=0.13 |
| Laboratory investigations | ||||
| White blood cells (×10^9/l) | 7 (5.3–14.8) | 6 (3.5–13.84) | 6.7 (3.7–7.5) | †P=0.10 |
| Red blood cells (×10^12/l) | 5 (3.8–5.3) | 4.6 (4.2–5.14) | 4.9 (4.24–5.47) | †P=0.18 |
| Haemoglobin (g/dl) | 11.8 (11.2–13.2) | 12.1 (9.9–13.4) | 12 (11.2–13.7) | †P=0.64 |
| Platelets (×10^9/l) | 268 (216–311) | 279.5 (239–313) | 285.5 (150–380) | †P=0.47 |
| T3 (ng/dl) | 111.5 (95–153) | 119.5 (94–177) | 118 (109–134) | †P=0.57 |
| T4 (µg/dl) | 8.1 (6.9–11.1) | 8.4 (6.8–10.8) | 8.3 (6.6–11.3) | †P=0.75 |
| Thyroid-stimulating hormone (µU/ml) | 1.5 (0.4–2.9) | 1.3 (0.4–3.78) | 1.6 (1.1–2.6) | †P=0.51 |
| Serum ferritin | 30.8 | 28.2 | 48.9 | †P=0.14 |
T3: Thyroid hormone 3, T4: Thyroid hormone 4, †: Kruskal Wallis, ‡: Chi-square test; §: Monte Carlo Exact P value. P<0.05 was significant. *: Statistically significant
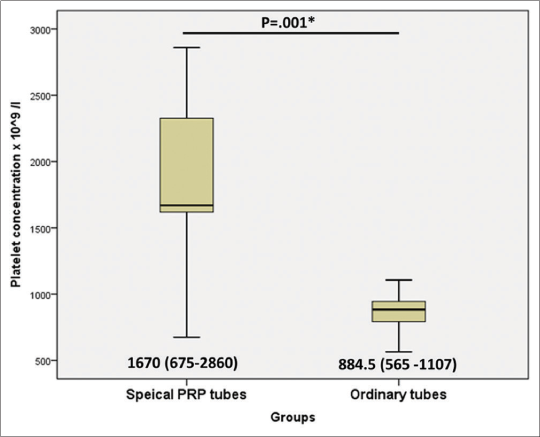
- Comparison between special platelet-rich plasma tubes and ordinary tubes groups as regards platelet concentration assessment
The hair pull test and visual analog scale results showed a significant difference between (group 1 vs.group 3) (P<0.001; P= 0.01) and (group 2 vs.group 3), (P= 0.001; P= 0.008) at 3 months after sessions, while there was no difference between (group 1 vs. group 2) as shown in [Table 2]. Trichoscopy results showed a significant increase in hair density in the frontal, temporal, and vertex areas after one and three months of the last session, between group 1 vs. group 3 and group 2 vs.group 3, while there was no difference between group 1 vs. group 2 as shown in [Table 2]. As regard hair thickness in the frontal, temporal and vertex areas, there was a significant increase in hair thickness in group 3 versus groups 1 and 2 before treatment. This significant difference becomes not evident one month and three months after treatment between (group 1 vs. group 3) and (group 2 vs. group 3) as shown in [Table 2].
| Group (1) | Group (2) | Group (3) | P1 | P2 | P3 | P-value | |
|---|---|---|---|---|---|---|---|
| (n = 10) | (n = 10) | (n = 10) | |||||
| Median | Median | Median | |||||
| (Min-Max) | (Min-Max) | (Min-Max) | |||||
| Hair pull test (number of pulled hair) Before treatment |
8.5 (7-12) | 9.5 (6-12) | 8 (6-12) | 0.5 | |||
| One month after last session | 4 (2-5) | 3 (2-5) | 7 (5-8) | 1 | <0.001* | 0.002* | <0.001* |
| Three months after last session | 2 (0-3) | 2 (0-4) | 6.5 (3-10) | 1 | 0.001* | 0.0001* | <0.001* |
| Visual analogue scale | |||||||
| Before treatment | 6 (5-6) | 6 (4-6) | 5.5 (4-6) | 0.5 | |||
| One month after last session | 4 (2-6) | 4 (1-5) | 5 (4-6) | 1 | 0.03* | 0.03* | 0.03* |
| Three months after last session | 3 (1-4) | 2.5 (1-5) | 5 (2-6) | 1 | 0.008* | 0.01* | 0.003* |
| Frontal hair trichoscopic examination | |||||||
| Before treatmentHair density/cm2 | 1076.4 (885.4-1388.9) | 1250 (815.97-1614.6) | 1116.8 (885.4-1579.87) | 0.1 | |||
| Hair thickness (mm) | 0.02 (0.02-0.03) | 0.018 (0.01-0.02) | 0.024 (0.02-0.03) | 0.6 | 0.002* | 0.03* | 0.003* |
| One month after last session Hair | 1536.4 (1180.5-2152.8) | 1666.7 (1354.17-1944.43) | 1076.4 (902.8-1545.13) | 0.6 | <0.001* | 0.03* | 0.001* |
| density/cm2 Hair thickness (mm) |
0.024 (0.02-0.03) | 0.023 (0.02-0.03) | 0.024 (0.02-0.03) | 0.7 | |||
| Three months after last session, | 1675.38 (1284.7-2500) | 1901.08 (1631.97-2343.77) | 1047.05 (902.77-1614.60) | 0.6 | <0.001* | 0.03* | <0.001* |
| Hair density/cm2 Hair thickness (mm) |
0.026 (0.02-0.03) | 0.026 (0.02-0.03) | 0.024 (0.02-0.03) | 0.3 | |||
| Temporal hair trichoscopic examination | |||||||
| Before treatment, Hair density/cm2 | 1015.7 (833.3-1406.3) | 1067.8 (781.3-1302.1) | 1119.8 (885.4-1562.5) | 0.5 | |||
| Hair thickness (mm) | 0.018 (0.01-0.03) | 0.016 (0.01-0.02) | 0.02 (0.02-0.03) | 1 | 0.001* | 0.01* | 0.01* |
| One month after last session, Hair | 1458.4 (1197.9-1875) | 1536.45 (1302.1-1822.9) | 1093.8 (781.3-1510.4) | 1 | 0.001* | 0.01* | 0.001* |
| density/cm2 Hair thickness (mm) |
0.023 (0.02-0.03) | 0.02 (0.02-0.03) | 0.023 (0.02-0.03) | 0.1 | |||
| Three months after last session, | 1770.85 (1406.3- | 1770.85 (1510.4-2291.7) | 1063.2 (729.2-1646.3) | 1 | 0.001* | 0.01* | <0.001* |
| Hair density/cm2 | 2187.5) | ||||||
| Hair thickness (mm) | 0.025 (0.02-0.03) | 0.02 (0.02-0.04) | 0.022 (0.02-0.03) | 0.1 | |||
| Vertex hair trichoscopic examination | |||||||
| Before treatment, Hair density/cm2 | 1093.8 (885.4-1770.8) | 1484.35 (625-1718.8) | 1145.8 (893.7-1770.8) | 0.3 | |||
| Hair thickness (mm) | 0.021 (0.02-0.03) | 0.019 (0.02-0.02) | 0.025 (0.02-0.03) | 0.5 | 0.001* | 0.03* | 0.001* |
| One month after last session, Hair | 1406.45 (1250-2083.3) | 1822.9 (1145.8-2291.7) | 1145.8 (989.60-1618.8) | 0.4 | 0.001* | 0.04* | 0.001* |
| density/cm2 Hair thickness (mm) |
0.026 (0.02-0.03) | 0.023 (0.02-0.03) | 0.024 (0.02-0.03) | 0.5 | |||
| Three months after last session, | 1666.7 (1458.3-2708.3) | 2083.3 (1406.3-2500) | 1067.75 (898.4-1614.6) | 0.5 | <0.001* | 0.01* | <0.001* |
| Hair density/cm2 Hair thickness (mm) |
0.026 (0.02-0.03) | 0.025 (0.01-0.03) | 0.024 (0.02-0.03) | 0.4 | |||
P-value: Significance between groups, P1: Significance between group 1 and 2, P2: Significance between group 2 and 3, P3: Significance between group 1 and 3, Significance between 2 groups assessed by Mann-Whitney U test; and between 3 groups by H: Kruskal-Wallis test, P < 0.05 was significant, *: Statistically significant
Comparison of frontal hair density and thickness (baseline, one month, three months of treatment) in each group, revealed a significant increase in groups 1 and 2 only (P<0.001) as shown in [Figure 3a]. Furthermore, there was a significant increase in temporal hair density and thickness (baseline, one month, three months of treatment) (P<0.001) in groups 1 and 2 only as shown in [Figure 3b]. Furthermore, the vertex hair density (baseline, one month, and three months of treatment) showed a significant increase in groups 1 and 2 (P<0.001) and a significant increase in hair thickness in groups 1(P<0.001) and 2 (P= 0.001) as shown in [Figure 3c]. A representative of global and trichoscopic pictures (×200) was shown in group 1 [Figure 4a], group 2 [Figure 4b], and group 3 [Figure 4c].

- Comparison as regards trichoscopic hair density and thickness in the frontal (a), temporal (b), and vertex areas (c) (×200)

- Global and trichoscopic (×200) pictures of the frontal area in a patient group 1 (a), group 2 (b), and group 3 (c)
The patient satisfaction showed a significant difference between (group 1 vs. group 3) (P<0.001) and (group 2 vs. group 3) (P<0.001), however, there was no difference between (group 1 vs. group 2) as shown in [Table 3]. As regards safety, there was no statistically significant difference between the 3 groups with regards to local side effects. In (group 1) one case (10%) reported a headache. There were no recorded systemic side effects in the three groups.
| Patient’s Satisfaction (3 month after last session) | Group (1) | Group (2) | Group (3) | P-value | |
|---|---|---|---|---|---|
| (n=10) | (n=10) | (n=10) | |||
| Median (Min-Max) | Median (Min-Max) | Median (Min-Max) | |||
| Since the start of the study; I can see the less | 1 (1-2) | 1 (1-2) | 4 (2-5) | <0.001* | |
| hairy areas in my hair get better | |||||
| P1 | 1 | ||||
| P2 | <0.001* | ||||
| P3 | <0.001* | ||||
| Because of the treatment I have received since | 1 (1-2) | 1 (1-2) | 4 (2-6) | <0.001* | |
| the start of the study; the appearance of my hair is | |||||
| P1 | 1 | ||||
| P2 | <0.001* | ||||
| P3 | <0.001* | ||||
| Since the start of the study; how would you | 1 (1-3) | 1 (1-2) | 4 (2-6) | <0.001* | |
| describe the growth of your hair | |||||
| P1 | 1 | ||||
| P2 | <0.001* | ||||
| P3 | <0.001* | ||||
| Since the start of the study; how effective do you | 1 (1-2) | 1 (1-2) | 3 (2-4) | <0.001* | |
| think the treatment has been in slowing down your hair loss | |||||
| P1 | 1 | ||||
| P2 | <0.001* | ||||
| P3 | <0.001* | ||||
| Compared to the beginning of the study; which statement best | 1 (1-2) | 1 (1-2) | 4 (2-5) | <0.001* | |
| describes your satisfaction with the appearance of frontal area | |||||
| P1 | 1 | ||||
| P2 | <0.001* | ||||
| P3 | <0.001* | ||||
| Compared to the beginning of the study; which statement best | 1 (1-1) | 1 (1-2) | 4 (2-5) | <0.001* | |
| describes your satisfaction with the appearance of temporal area | |||||
| P1 | 1 | ||||
| P2 | <0.001* | ||||
| P3 | <0.001* | ||||
| Compared to the beginning of the study; which statement best | 1 (1-2) | 1 (1-2) | 4 (2-5) | <0.001* | |
| describes your satisfaction with the appearance of overall hair | |||||
| P1 | 1 | ||||
| P2 | <0.001* | ||||
| P3 | <0.001* | ||||
P: Significance between groups, P1: Significance between group 1 and group 2, P2: Significance between group 2 and group 3, P3: Significance between group 1 and group 3, Significance between 2 groups assessed by Mann-Whitney U test, and between 3 groups by Kruskal-Wallis test, P<0.05 was significant,*: Statistically significant
There was a significant positive correlation between baseline platelet concentration and platelet concentration in the platelet-rich plasma (r = 0.483, P = 0.03). However, there was no significant correlation between platelet concentration and age nor any of the hair evaluating tools as shown in [Table 4].
| Baseline platelets | Age | Hair pull test | Visual analogue scale | Frontal Hair density | Frontal hair thickness | Temporal Hair density | Temporal hair thickness | |
|---|---|---|---|---|---|---|---|---|
| Group 1+Group 2(n=20) | ||||||||
| Platelets Conc. | ||||||||
| r | 0.483 | –0.05 | –0.14 | –0.029 | –0.279 | 0.433 | 0.421 | 0.133 |
| P | 0.03* | 0.834 | 0.55 | 0.91 | 0.234 | 0.057 | 0.07 | 0.57 |
| Group 1(n=10) | ||||||||
| Platelets Conc. | ||||||||
| r | 0.96 | –0.702 | –0.184 | –0.196 | –0.048 | 0.540 | 0.475 | 0.478 |
| P | 0.0001* | 0.024 | 0.610 | 0.587 | 0.894 | 0.107 | 0.17 | 0.16 |
| Group 2(n=10) | ||||||||
| Platelets Conc. | ||||||||
| r | 0.973 | –0.572 | –0.284 | 0.127 | 0.107 | –0.343 | –0.4 | 0.464 |
| P | 0.0001* | 0.084 | 0.43 | 0.726 | 0.77 | 0.33 | 0.25 | 0.18 |
r: Pearson correlation (for all data except visual analogue scale where Spearman correlation was used), P<0.05 was significant, *: Statistically significant
Discussion
The current study demonstrated the efficacy of platelet-rich plasma (both special platelet-rich plasma tubes and ordinary tubes groups) in the treatment of chronic telogen effluvium in comparison to control. Platelet-rich plasma-treated patients showed improvement of visual analog scale, hair pull test, and trichoscopic hair density and thickness after 1 month and 4 monthly sessions. Moreover, this improvement was maintained up to three months of follow up. We were unable to find any clinical trials of treatment with platelet-rich plasma in chronic telogen effluvium. Our results are supported by a recent report in a single patient with chronic telogen effluvium. There was a significant increase in trichoscopic hair count and diameter after 3 monthly sessions of platelet-rich plasma combined with a high protein diet.14
Various mechanisms are supporting the positive effects of platelet-rich plasma on hair. Various growth factors, cytokines, and chemokines are released from platelet-rich plasma.7 These growth factors could act by activating the protein kinase B pathway,15 leading to increased proliferation of the hair follicle dermal papilla cells;16 and increased proliferation of hair follicle bulge cells as revealed by the increased Ki-67 which is a marker of cell proliferation. Furthermore, platelet-rich plasma could prevent hair follicle apoptosis by increasing levels of the anti-apoptotic protein Bcl-2.17
The reduction of pulled hair in the current study was (47–68%) in both platelet-rich plasma groups after 1 month and increased to 76% after three months. This finding is consistent with many of platelet-rich plasma clinical trials that have been conducted on androgenetic alopecia patients.8,18 The number of pulled hair was reduced by an average of 65% in androgenetic alopecia patients.19
In the current study, the median of hair density is significantly increased in the frontal, temporal, vertex hair by (55.61, 74.3, and 52.4% in the special tubes group), (52.1, 65.8, and 40.4% in the ordinary tubes group) respectively median of hair thickness is increased significantly in the frontal, temporal, vertex hair by (30, 38.9, and 23.8% in special tubes group), (44.4, 25, and 31.6% in ordinary tubes group) respectively. So, the percentage of improvement in hair density was more than the hair thickness. This trichoscopic improvement has been reported in a patient with chronic telogen effluvium treated with platelet-rich plasma resulting in increased hair thickness and count by 11%.14 This improvement percentage is lower than our result which could be due to a lesser number of sessions.
Furthermore, this trichoscopic hair improvement has been reported in the literature in several studies of androgenetic alopecia patients. The mean increase of hair density in platelet-rich plasma treated androgenetic alopecia patients and control, was 77.28, 17.81 hair/cm2, respectively. Furthermore, the hair thickness significantly improved in platelet-rich plasma-treated patients (0.11mm) versus control (0.03mm).18 In another study, there was a significant increase in hair count and density.20 In a more recent study only hair density increased compared to control.
In the current study, the trichoscopic hair improvement was maintained up to three months which is consistent with studies in androgenetic alopecia. Platelet-rich plasma therapy significantly improved mean hair count, and thickness as compared to baseline after three months (20.5 ± 17%, 31.3 ± 30.1%) respectively; and this improvement was maintained after six months (29.2 ± 17.8%, 46.4 ± 37.5%) respectively.21 In another randomised controlled trial, there was a significant increase in hair density (31%) in comparison to control (1%) after three months, however, this study did not evaluate hair thickness.22 The longer follow-up of androgenetic alopecia patients one year after platelet-rich plasma therapy showed a significant increase of hair density (154.8 ± 34.3, 170.7 ± 37.8, 156.2 ± 37.7, and 153.7 ± 39.9 hair/cm2 respectively in 1.5, 3, 6, 12 months). However, this study claimed that platelet-rich plasma leads to more improvement in hair diameter than hair count.23 Therefore, there are variable effects of platelet-rich plasma therapy on hair density and thickness. In the current study, the improvement of both hair density and thickness was noticed. This variability could be due to different platelet-rich plasma preparations and different set of patients as compared to our study.
The current study showed no statistically significant difference between the three groups as regards local side effects. In group 1, one case (10%) reported headache. These results indicate good safety profile of platelet-rich plasma therapy as reported in patients with androgenetic alopecia.19,24,25 Rare incidence of other side effects have been reported such as pruritus and dermatitis26 and alopecia with psoriasis form scalp dermatitis 6 weeks after platelet-rich plasma.27
The current study compared two methods of platelet-rich plasma preparations. Although the special platelet-rich plasma tubes showed significantly higher platelet concentration (4.0– 7.8 fold) in comparison to the ordinary tubes (2.5–3.1 fold), there was no significant difference in the hair improvement parameters. The platelet concentration using the special platelet-rich plasma tubes in the current study is comparable to what has been reported in other studies as ranged from 5.9 folds;28 to 4-7 folds.19 In a review analyzing 12 studies, the platelet concentration ranged from 2.6 to 7.3 folds. It has been suggested that four folds increase of the platelet concentration is considered optimal for efficacy in androgenetic alopecia.29 Hair assessment tools have shown significant improvement in various studies with both low and high platelet concentrations.30,31
The current study has several limitations. The sample size was small (ten patients/group). The follow-up of patients was three months only. Therefore, a multi-centre randomised controlled clinical study with a large sample size and longer follow-up is recommended. Furthermore, studies using different types of platelet-rich plasma tubes also needs to be done. It will be interesting to compare platelet-rich plasma injections using syringe versus derma pen in patients with chronic telogen effluvium.
Conclusion
Platelet-rich plasma could be considered as a promising therapy for chronic telogen effluvium patients with an excellent safety profile. The ordinary laboratory low-cost tubes might be a reliable alternative to the expensive special PRP kits tubes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Diffuse hair loss in an adult female: Approach to diagnosis and management. Indian J Dermatol Venereol Leprol. 2009;75:20-7. quiz 27-8
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic chronic telegon effluvium in the woman. Hautarzt. 2000;51:899-905.
- [CrossRef] [PubMed] [Google Scholar]
- Telogen effluvium. Indian J Dermatol Venereol Leprol. 2013;79:591-603.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma in dermatology: Boon or a bane? Indian J Dermatol Venereol Leprol. 2014;80:5-14.
- [CrossRef] [PubMed] [Google Scholar]
- Principles and methods of preparation of platelet-rich plasma: A review and author's perspective. J Cutan Aesthet Surg. 2014;7:189-97.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized Placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-7.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma in androgenic alopecia: Myth or an effective tool. J Cutan Aesthet Surg. 2014;7:107-10.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of platelet-rich plasma for androgenetic alopecia: A review of the literature. Skin Appendage Disord. 2018;4:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Hair evaluation methods: Merits and demerits. Int J Trichol. 2009;1:108-19.
- [CrossRef] [PubMed] [Google Scholar]
- A hair growth questionnaire for use in the evaluation of therapeutic effects in men. J Dermatol Treatment. 1998;9:181-6.
- [CrossRef] [Google Scholar]
- Adenosine increases anagen hair growth and thick hairs in Japanese women with female pattern hair loss: a pilot, double-blind, randomized, placebo-controlled trial. J Dermatol. 2008;35:763-7.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma-an “Elixir” for treatment of alopecia: Personal experience on 117 patients with review of literature. Stem Cell Investig. 2017;4:64.
- [CrossRef] [PubMed] [Google Scholar]
- A mechanistic model of platelet-rich plasma treatment for androgenetic alopecia. Dermatol Surg. 2016;42:1335-9.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous platelet-rich plasma: A potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012;387(Pt 1):1040-6.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of autologous activated platelet-rich plasma injection on female pattern hair loss: A randomized placebo-controlled study. J Cosmet Dermatol. 2018;17:47-53.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of platelet-rich plasma in treatment of androgenic alopecia. Asian J Transfus Sci. 2015;9:159-62.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of platelet-rich plasma in hair regrowth: A randomized placebo-controlled trial. Stem Cells Transl Med. 2015;4:1317-23.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of autologous activated platelet rich plasma (AAPRP) injection on pattern hair loss: Clinical and histomorphometric evaluation. Biomed Res Int. 2014;2014:760709.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of not-activated and activated prp in hair loss treatment: Role of growth factor and cytokine concentrations obtained by different collection systems. Int J Mol Sci. 2017;18:408.
- [CrossRef] [PubMed] [Google Scholar]
- Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-9.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis of efficacy of platelet-rich plasma therapy for androgenetic alopecia. J Dermatol Treat. 2017;28:55-8.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma for the treatment of female pattern hair loss: A patient survey. Dermatol Surg. 2018;44:130-2.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized, placebo-controlled trial of 1% topical minoxidil solution in the treatment of androgenetic alopecia in Japanese women. Eur J Dermatol. 2007;17:37-44.
- [Google Scholar]
- New investigational drugs for androgenetic alopecia. Expert Opin Investig Drugs. 2013;22:573-89.
- [CrossRef] [PubMed] [Google Scholar]
- Combination of a non-ablative 1,927 nm thulium fiber fractional laser and autologous platelet-rich plasma in treatment of male androgenetic alopecia: A pilot study. Chulalongkorn Med J. 2019;63:13-21.
- [Google Scholar]
- A study to compare the efficacy of platelet-rich plasma and minoxidil therapy for the treatment of androgenetic alopecia. Int J Trichol. 2019;11:68-79.
- [CrossRef] [PubMed] [Google Scholar]
- A proposal of an effective platelet-rich plasma protocol for the treatment of androgenetic alopecia. Int J Trichol. 2017;9:165.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanical and controlled PRP injections in patients affected by androgenetic alopecia. J Vis Exp. 2018;131:56406.
- [CrossRef] [PubMed] [Google Scholar]






