Translate this page into:
Histological analysis of different types of port-wine stains to guide clinical decision making: A retrospective study
Corresponding author: Prof Xian Jiang, Department of Dermatology, Laboratory of Dermatology, Clinical Institute of Inflammation and Immunology (CIII), Frontiers Science Center for Disease-related Molecular Network, West China Hospital, Sichuan University, 37 Guoxue Alley, Wuhou District, Chengdu, Sichuan 610041, China. jennyxianj@163.com
-
Received: ,
Accepted: ,
How to cite this article: Liu L, Zhou L, Zhao Q, Li X, Yang L, Li E, et al. Histological analysis of different types of port-wine stains to guide clinical decision making: A retrospective study. Indian J Dermatol Venereol Leprol 2023;89:204-12.
Abstract
Background and objectives:
Port-wine stains are defined as congenital benign vascular lesions. The treatment of port-wine stains remains a challenge, worldwide. This study aimed to analyze the histological characteristics in different types of port-wine stains and provide guidance for clinical decision-making.
Methods and materials:
Biopsies were from the hospital from 2015 to 2021. H&E staining, Immunofluorescence staining, Masson’s trichrome staining and Weigert staining were performed on the tissues.
Results:
A total of 35 port-wine stains patients were included in the study of four distinct types, namely red port-wine stains (11 cases), purple port-wine stains (seven cases), hypertrophic port-wine stains (nine cases) and nodular port-wine stains (eight cases). The mean vessel diameter of the different types was 38.7 ± 5.9 μm, 93.5 ± 9.7 μm, 155.6 ± 21.8 μm and 155.6 ± 29.54 μm, respectively. Mean vessel depth was 396.4 ± 31 μm, 944.2 ± 105.4 μm, 2,971 ± 161.3 μm and 3,594 ± 364.6 μm, respectively. The vessels in red port-wine stains, purple port-wine stains and hypertrophic port-wine stains were mainly composed of capillary and venous malformations, whereas those in nodular port-wine stains were venous or arteriovenous malformations.
Limitation:
The main limitation of the current study was the small number of patients.
Conclusion:
As the disease progresses, vessel diameters become larger, the vessel wall becomes thicker and vessels were found in a greater depth. A treatment plan should be scientifically formulated keeping in mind the histological characteristics of port-wine stains.
Keywords
Laser
port-wine stains
vascular malformations
vessel diameter
vessel depth
Plain Language Summary
Port-wine stains are defined as congenital benign vascular malformations and the treatment of port-wine stains remains a challenge worldwide. As is well known, the treatment efficiency could be influenced by multiple factors such as age, location, skin color, etc. However, it is substantially related to the pathological characteristics of port-wine stains: vessel diameter, vessel depth, vessel density, vessel wall thickness and fibers in the lesions. The aim of our study was to characterize the histological characteristics of different types of port-wine stains. As the disease progressed, vessel diameters became larger, the vessel wall became thicker and vessels were found in greater depth. In addition, the types of vessels changed, venous malformations gradually increased, and arteriovenous malformations and tissue hyperplasia occurred, further increasing the difficulty of treatment. Hence a treatment plan should be scientifically selected with regards to the pathological characteristics of port-wine stains.
Introduction
Port-wine stains are congenital vascular malformations with an incidence of 3–5 per 1000 neonates.1 There are approximately 25 million patients worldwide.2 To date, the pulsed-dye laser is the gold standard for the treatment of port-wine stains. However, complete clearance is achieved in less than 10% of patients by pulsed-dye laser.3 Moreover in 16 to 50% of patients reappearance occurs after pulsed-dye laser treatment.4,5
Despite the availability of multiple treatment options, the treatment of port-wine stains remains a worldwide problem.6 As is well known, the treatment efficiency could be influenced by multiple factors such as age, location, skin colour, etc.7 It is also related to the pathological characteristics of port-wine stains: vessel diameter, vessel depth, vessel density, vessel wall thickness and fibres in the lesions.
According to literature, port-wine stains can be classified as flat and hypertrophic, like pink, red, purple or dark purple depending on the thickness and colour of the lesions.8,9 In the present study, port-wine stains were classified into four types with reference to previous studies: red, purple, hypertrophic and nodular port-wine stains.10,11 A previous study revealed that port-wine stains may not only be a congenital disease involving vessels, but also involve other tissue components in the skin.12 Till now, no comparative study has been conducted on the histological characteristics of different types of port-wine stains.
The aim of the present study was to characterize the histological characteristics and enhance our understanding of different types of port-wine stains. Furthermore, this could provide a new direction for treatment and explain the reasons for resistance to various therapies.
Methods and Materials
We retrospectively reviewed cases of port-wine stains from the archives of the department of dermatology in China, between 2015 and 2021. This study was approved by the Ethics Committee of West China Hospital of Sichuan University. Informed consent was obtained from the patient. The inclusion criteria were as follows: (a) Cutaneous lesions at birth; (b) No history of the treatment of the condition; (c) No port-wine stain-associated syndromes; (d) Clinical images confirming port-wine stains; (e) Histological confirmation. In total, 35 paraffin blocks of port-wine stain specimens and five of normal skin (tissue from around excised nevi or dermatofibroma) were included in the study. The clinical data of the patients were recorded.
The classification criteria were as follows [Figure 1]: (a) Red port-wine stains: pink, red or dark red patches; (b) Purple port-wine stains: purple or reddish-purple patches; (c) Hypertrophic port-wine stains: irregular red or purple plaques; (d) Nodular port-wine stains: Nodules that were red, dark red or grey-white. Vessel diameter and vessel depth were analysed from histological sections stained with hematoxylin and eosin. A total of ten vessels were randomly selected for measurement of the mean vessel diameter and depth. Sections from each group were stained for identification of the types of vessels, and the arrangement of collagen and elastic fibres using immunofluorescent, Masson’s trichrome and Weigert elastic fibre staining.
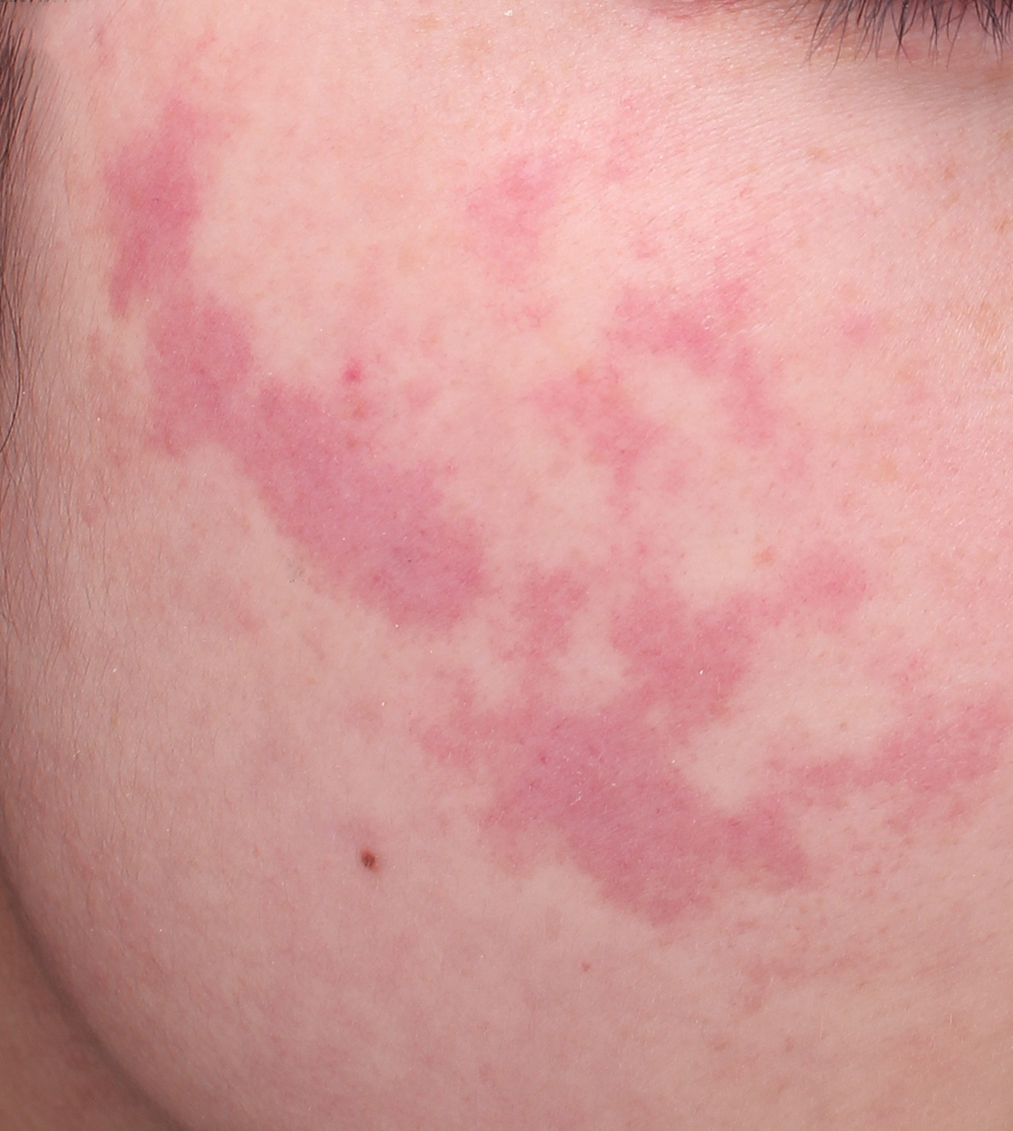
- Red port-wine stains: irregular pink patches
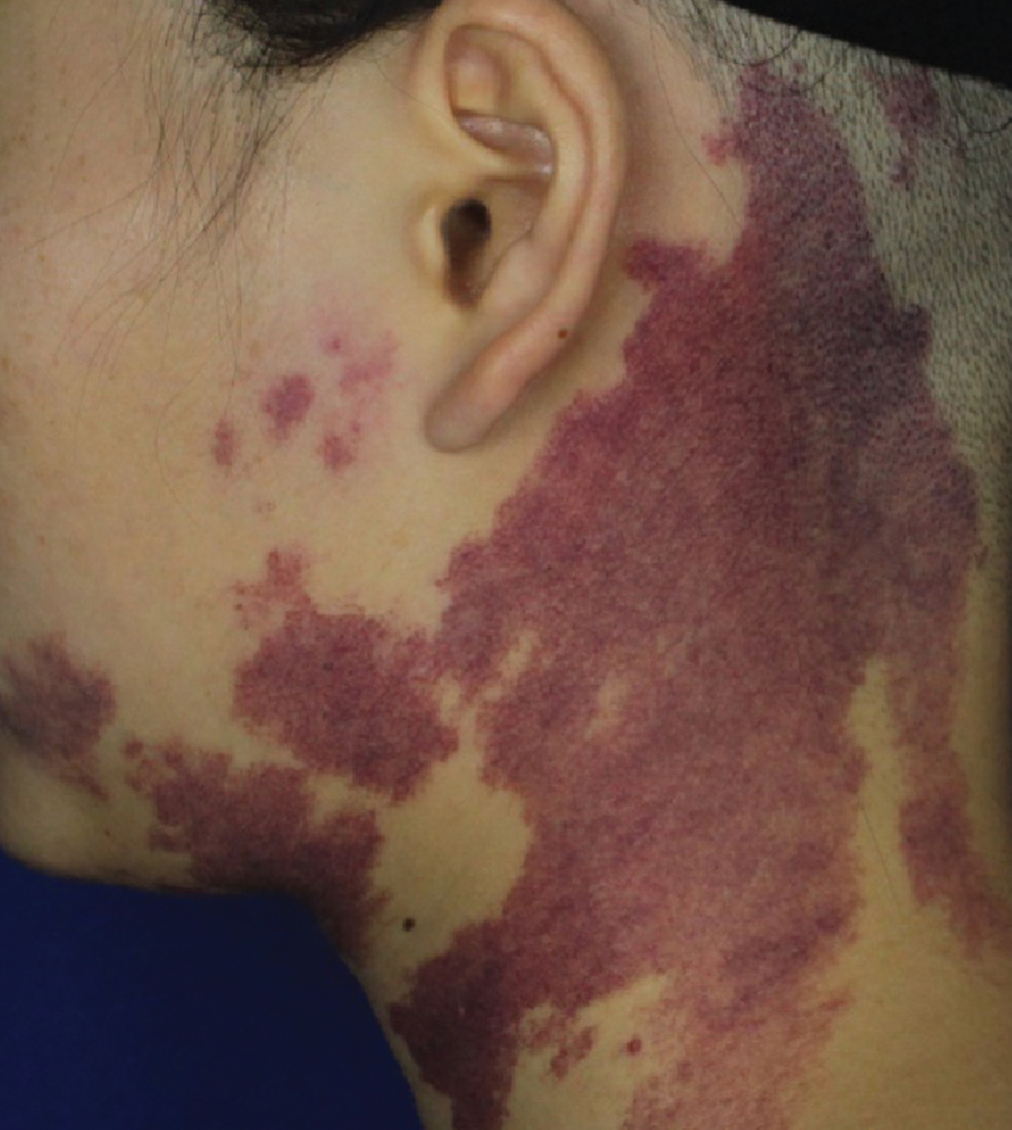
- Purple port-wine stains: irregular purplish-red patches with well-demarcated borders
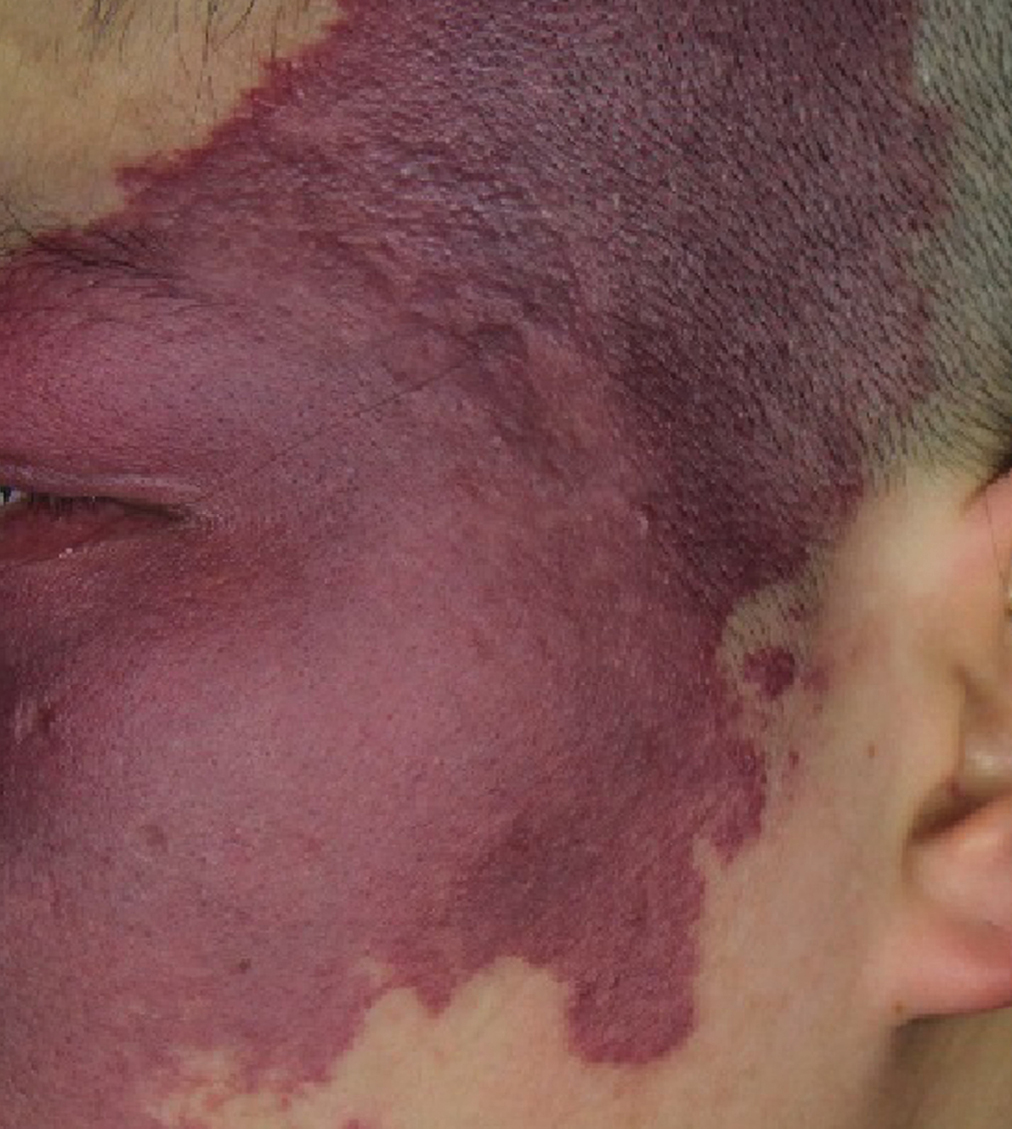
- Hypertrophic port-wine stains: irregular purplish-red plaques with well-demarcated borders
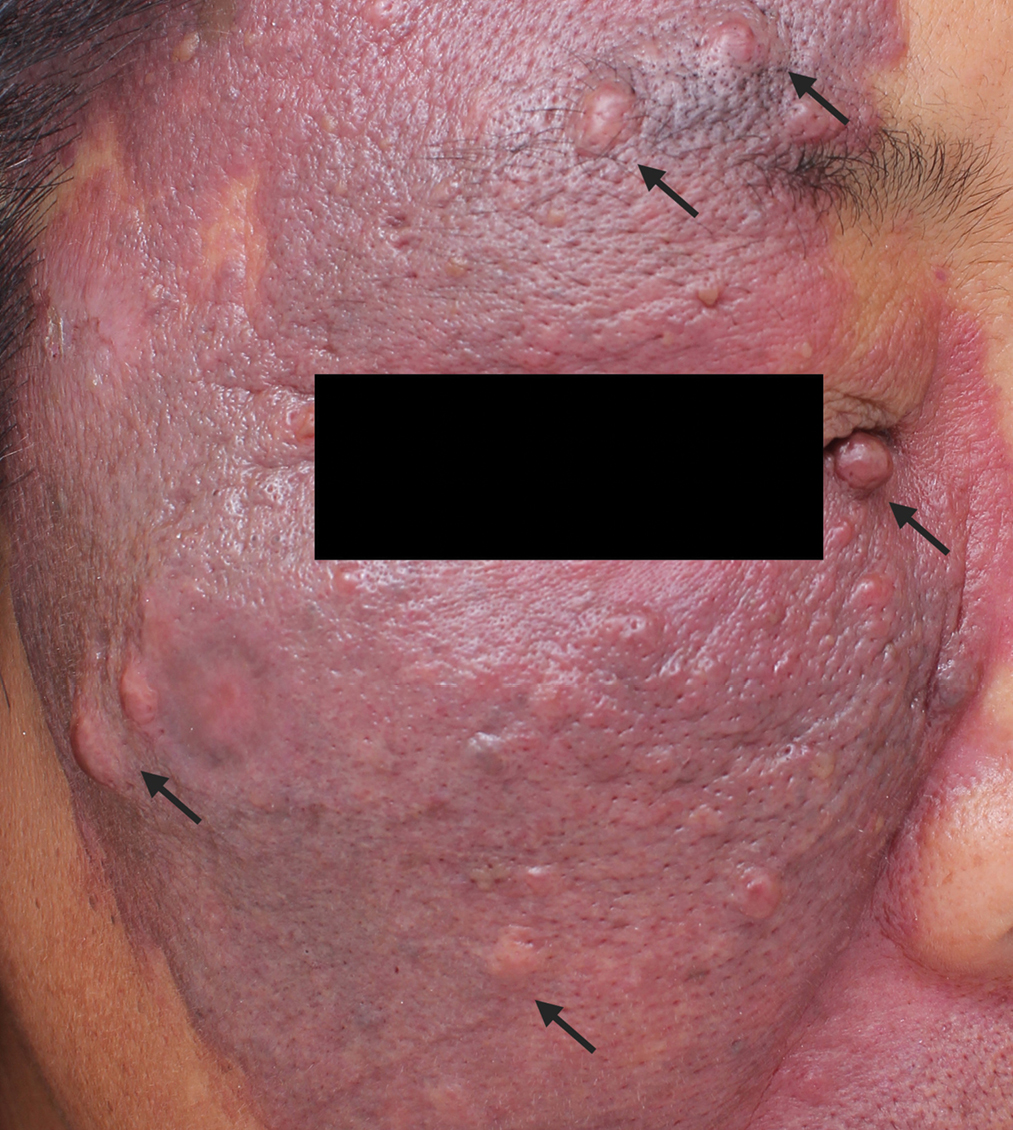
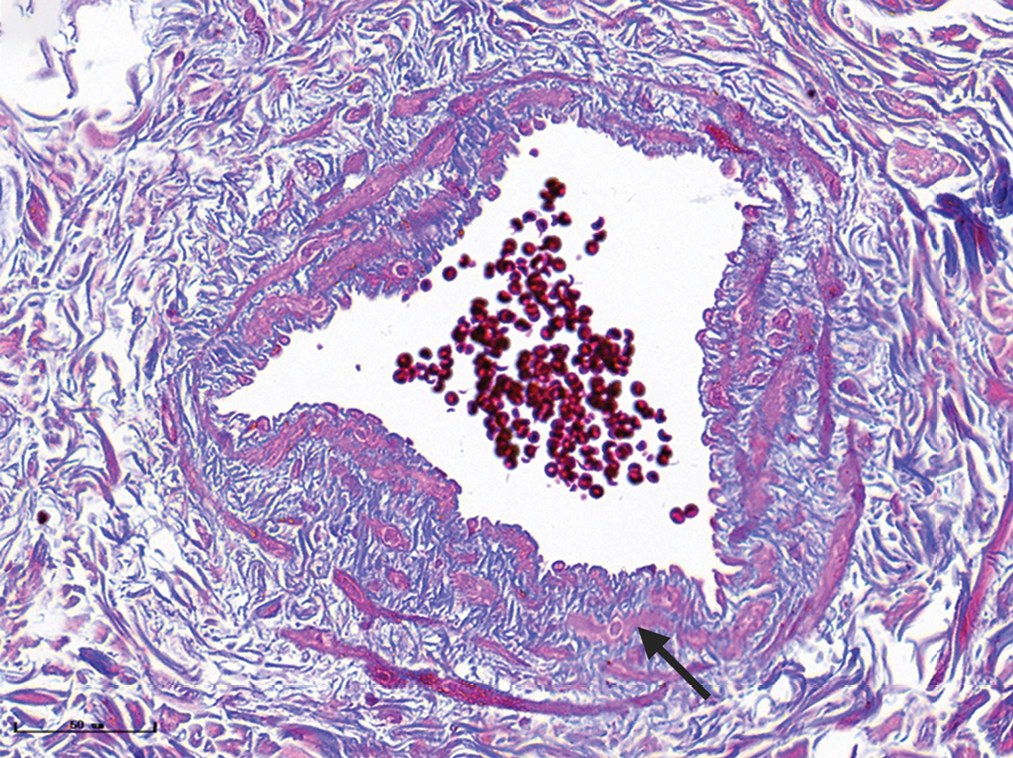
- Nodular port-wine stains: mung bean to pea-sized red nodules (black arrows) on the dark purplish-red plaques with clear borders and irregular shapes
Statistical analysis
The data were statistically analysed using SPSS v23.0 and GraphPad Prism 7.0 software. The results were expressed as arithmetic means ± SD. One-way analysis of variance was used for data analysis.
Results
Epidemiological and clinical features
The clinical features of the study population were summarized. There were 18 males and 17 females. The mean age of the patients was 33 years (median age: 29 years; range: 9–55 years). In all the case, the lesions were present from birth and were mostly located on the head and neck without a family history. Clinically, characteristics of the cutaneous lesions included pink, red, purple or reddish-purple patches, red or purple plaques, and various-sized nodules. There were 11 (31%) cases of red port-wine stains, seven (20%) purple port-wine stains, nine (35%) of hypertrophic port-wine stains and eight (23%) of nodular port-wine stains included.
Differences in depth and diameter of vessels in different types of port-wine stains
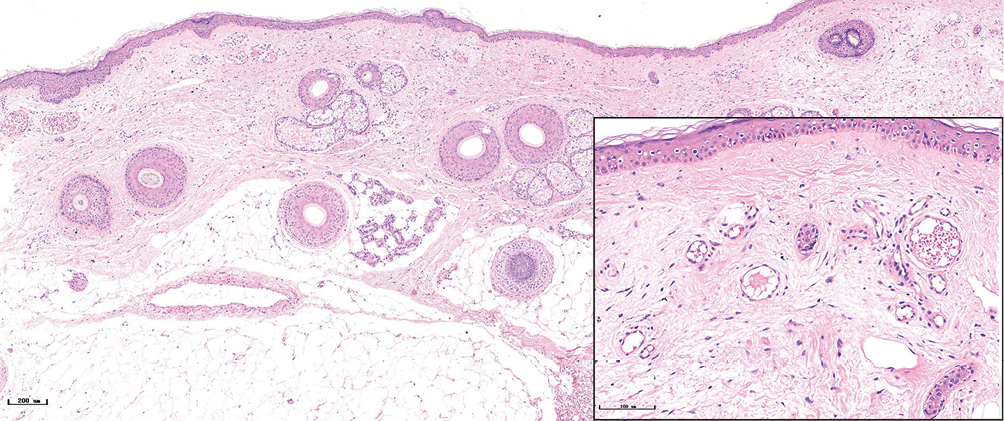
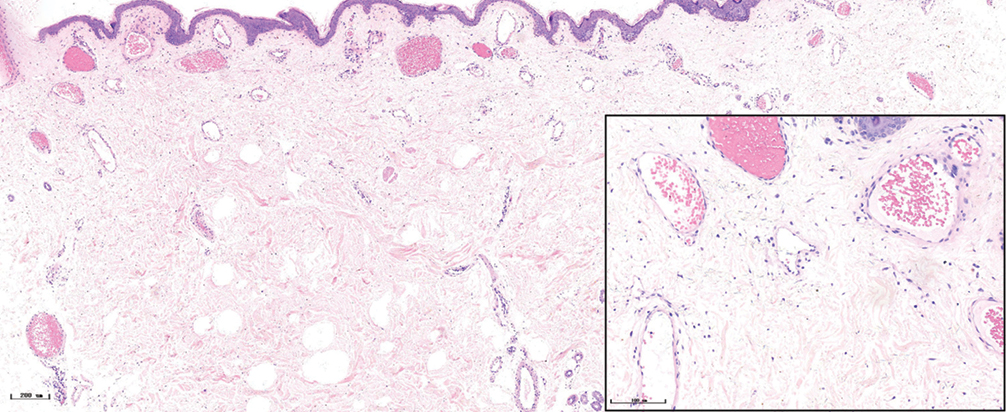
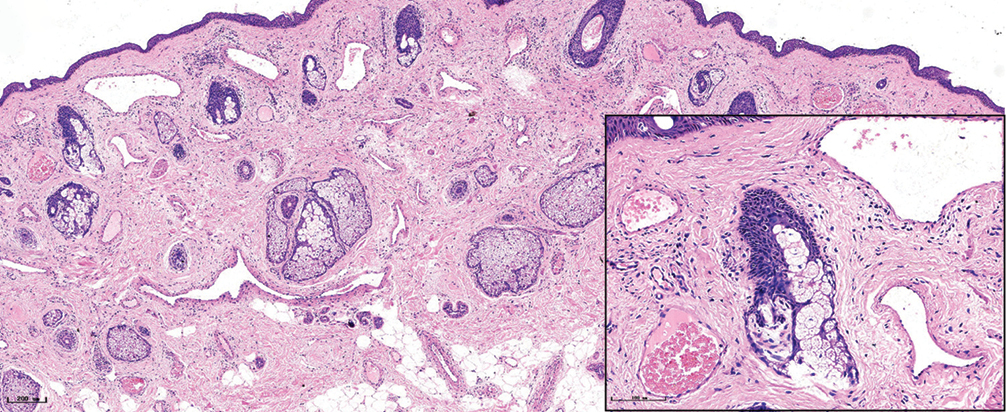
The histopathological features of the normal tissue and different types of port-wine stains are shown in Figure 2 and Table 1. The mean vessel diameter of the different types of port-wine stains were 38.7 ± 5.9 μm, 93.5 ± 9.7 μm, 155.6 ± 21.8 μm and 155.6 ± 29.54 μm, respectively. The mean vessel depth was 396.4 ± 31 μm, 944.2 ± 105.4 μm, 2,971 ± 161.3 μm and 3,594 ± 364.6 μm, respectively. Vessels from normal skin were scattered around the dermis. A small number of dilated vessels were visible in the dermis of red port-wine stains. The vessels were filled with red blood cells and the vascular walls were thin. The number of dilated vessels were greater in purple port-wine stains, which were mostly located in the superficial and medium dermis, with a small number of vessels located in the deep dermis. Blood vessel walls were slightly thickened. The number of dilated vessels were significantly greater and the vessel walls thicker still in hypertrophic port-wine stains. The depth of vessels was greater, distributed within the dermis and subcutaneous tissues. A large number of dilated vessels were visible throughout the dermis and subcutaneous tissue, while the thickness of the vessel walls was increased in nodular port-wine stains.
| Type | N | Mean vessel diameter (Mean ± SD) | Vessel diameter (Max) | Mean vessel depth (Mean ± SD) | Vessel depth (Max) |
|---|---|---|---|---|---|
| Normal subject | 10 | 11 ± 0.7 | 15 | No | No |
| Red PWS | 11 | 38.7 ± 5.9 | 91.9 | 396.4 ± 31 | 513.5 |
| Purple PWS | 7 | 93.5 ± 9.7 | 216.6 | 944.2 ± 105.4 | 1369.3 |
| Hypertrophic PWS | 9 | 155.6 ± 21.8 | 296.8 | 2971 ± 161.3 | 3474.3 |
| Nodular PWS | 8 | 155.6 ± 29.54 | 609.9 | 3594 ± 364.6 | 6540.2 |
No: The depth of vessels in normal subject were not measured, because the vessels spread throughout the dermis and were not dilated, PWS: port-wine stains
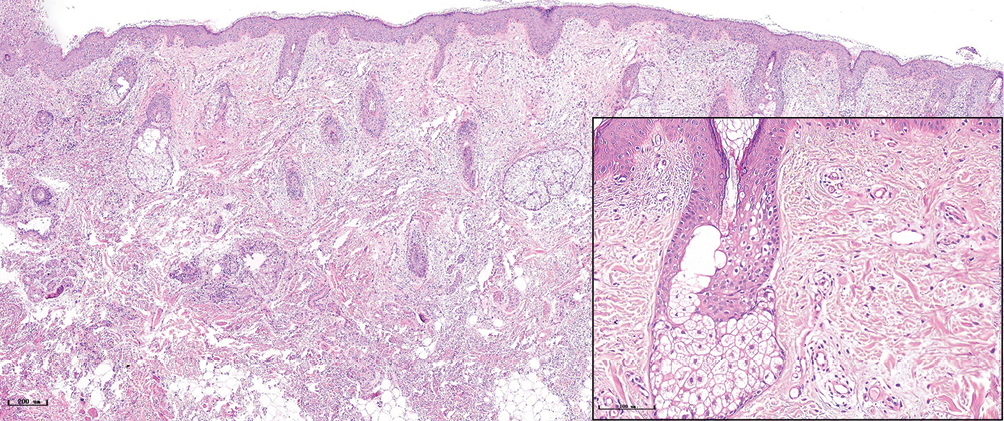
- Normal subject (H&E, ×50), black panel shows an enlargement (H&E, ×200)
- Red port-wine stains, several dilated vessels could be seen in the superficial dermis, and a few red blood cells could be seen in some vessels (H&E, ×50), black panel shows an enlargement (H&E, ×200)
- Purple port-wine stains, lots of dilated vessels filled with red blood cells in the superficial and middle dermis (H&E, ×50), black panel shows an enlargement (H&E, ×200)
- Hypertrophic port-wine stains, lots of dilated vessels in the whole dermis and subcutaneous tissue (H&E, ×50), black panel shows an enlargement (H&E, ×200)
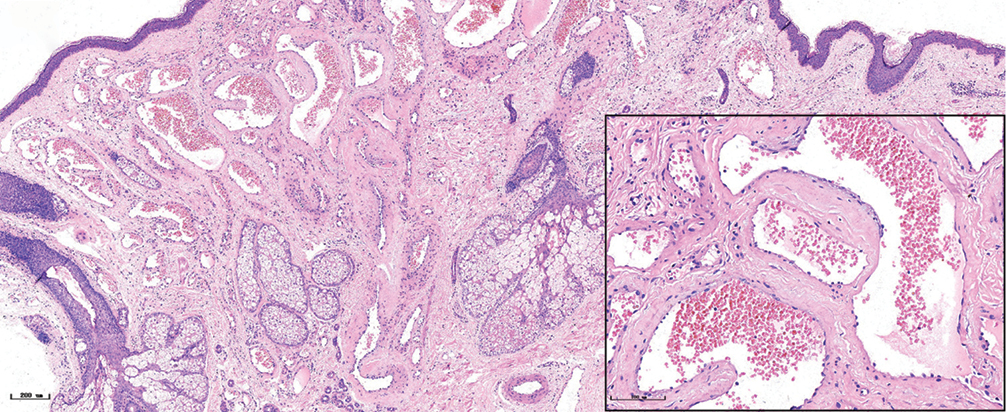
- Nodular port-wine stains, a large number of dilated vessels in the full dermis and subcutaneous tissue, with a significant number of red blood cells could be seen (H&E, ×50), black panel shows an enlargement (H&E, ×200).
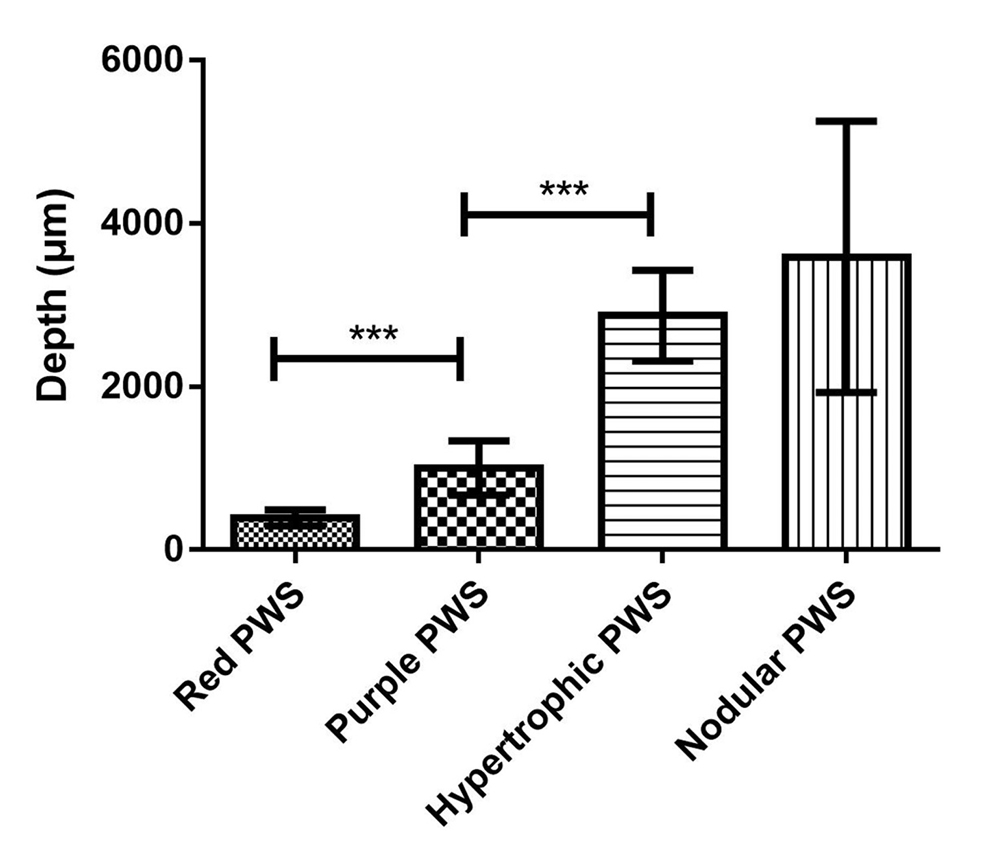
- Comparison of vessel depth in different types of port-wine stains, ***P <0.001
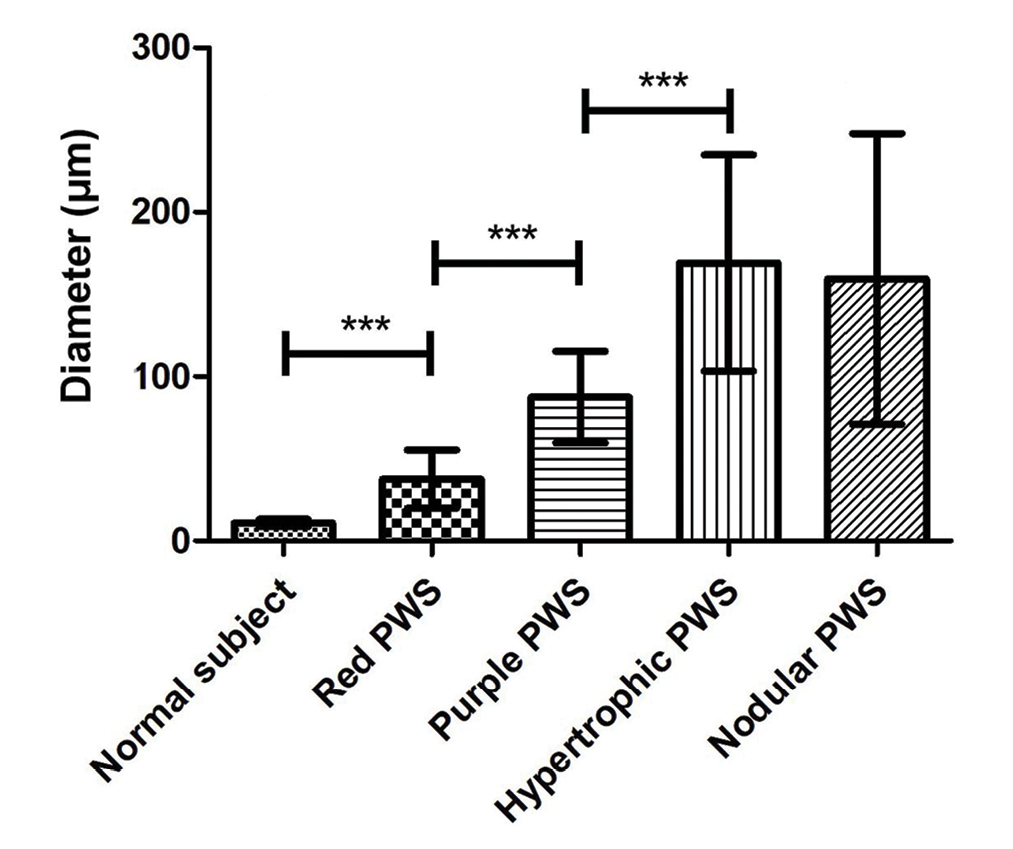
- Comparison of vessel diameter in different types of port-wine stains, ***P <0.001
Capillary malformations, venous malformations and arteriovenous malformations appeared in port-wine stains
The characteristics of vessels were identified using immunofluorescent staining, (Figure 3) (green: CD31, a marker for vascular endothelial cells; red: αSMA, a marker for pericytes and vascular smooth muscle cells; blue: nuclei). The expression of CD31 and αSMA in port-wine stains was higher than those in normal skin, while vessels were clearly dilated. The expression of αSMA in hypertrophic and nodular types [Figure 3] was significantly greater than in red port-wine stains and purple port-wine stains. Multi-layer green fluorescence surrounding the red fluorescence revealed that veins or arteries might be involved in the development of port-wine stains.
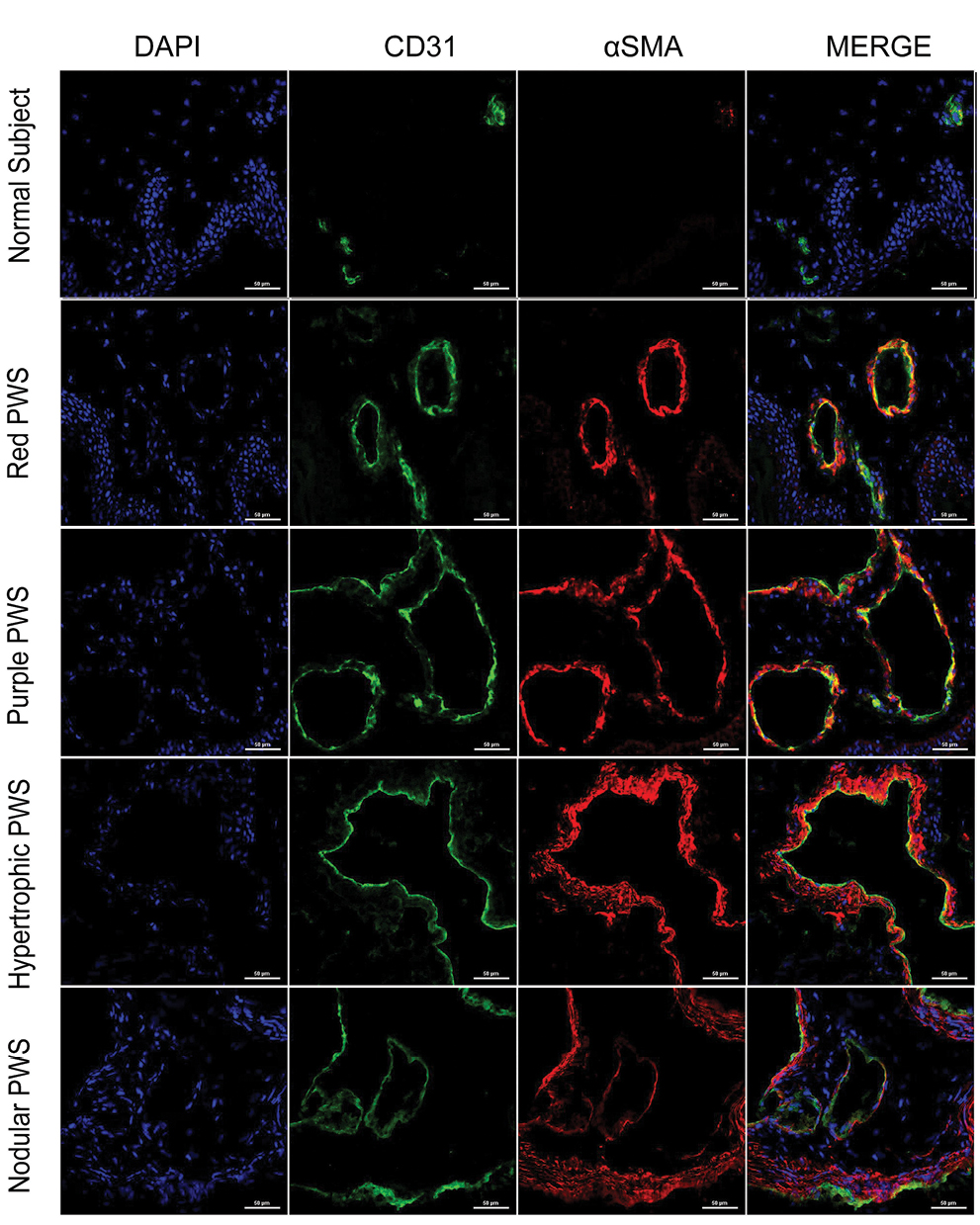
- Immunofluorescence staining of vascular endothelial cells, pericytes, and vascular smooth muscle cells in different types of port-wine stains. Green fluorescence indicates vascular endothelial cells (CD31 staining). Red fluorescence indicates vascular smooth muscle cells (αSMA staining). The vessels were slightly dilated and red fluorescence surrounded green fluorescence in red port-wine stains. The vessels in purple port-wine stains were more dilated than the vessels in red port-wine stains, and the fluorescence of αSMA and CD31 were equivalent. The vessels were significantly dilated and several layers of αSMA fluorescent structure appeared in hypertrophic port-wine stains and nodular port-wine stains (Immunofluorescence staining, ×400)
Changes in collagen and elastic fibres in different types of port-wine stains
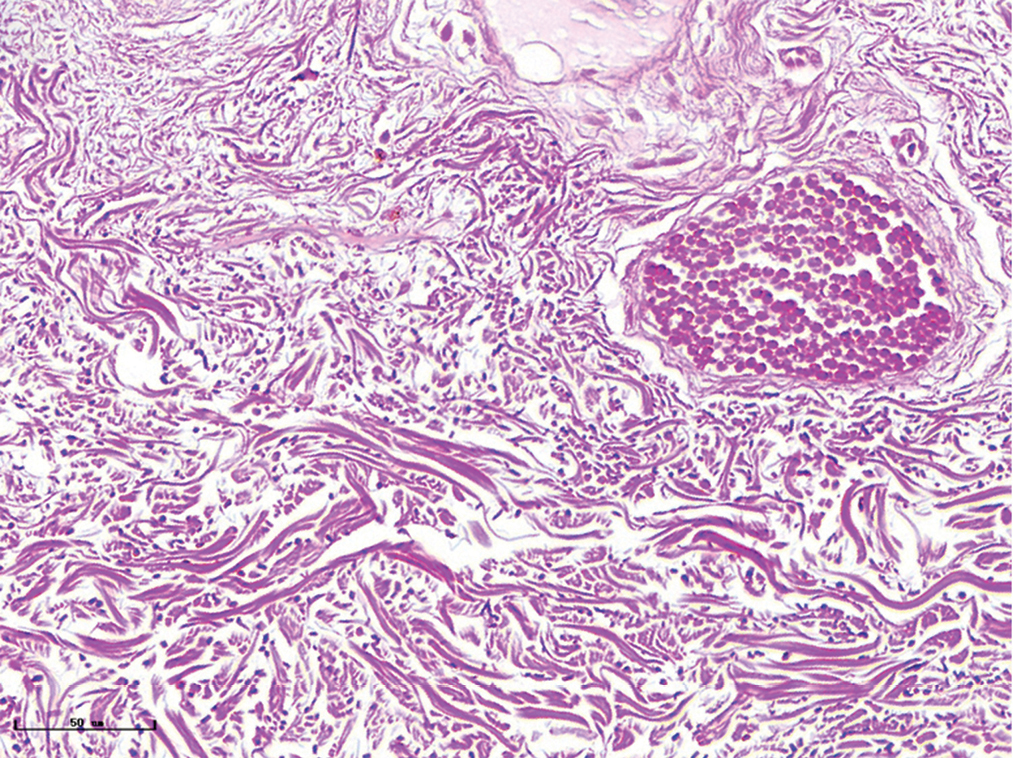
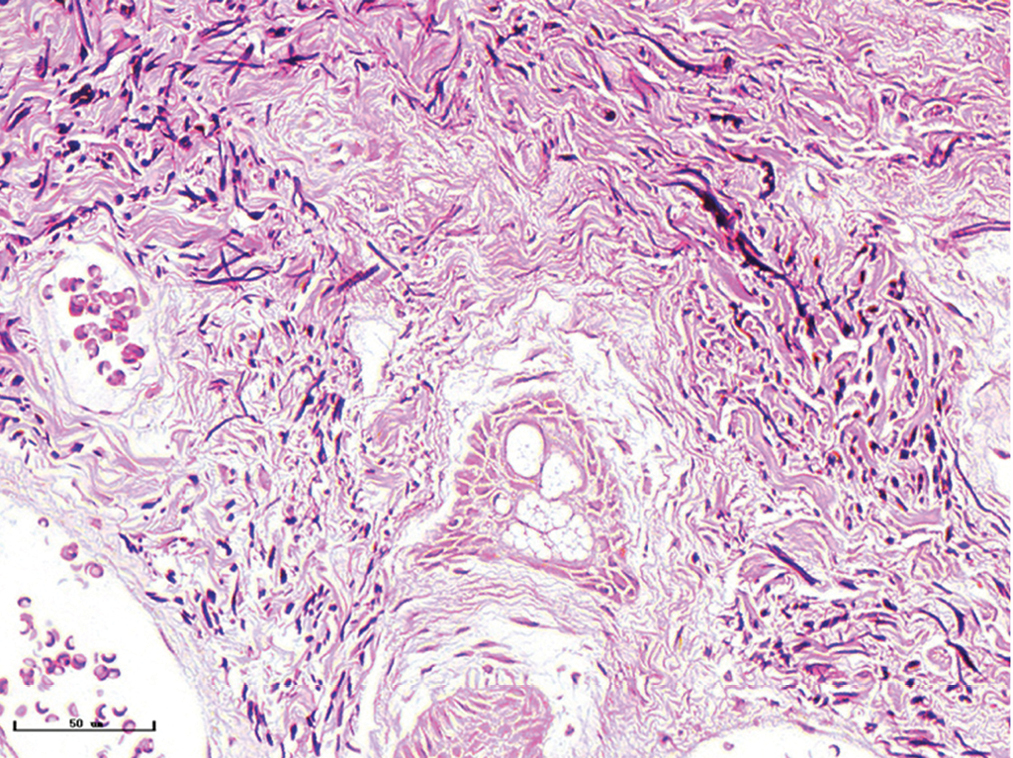
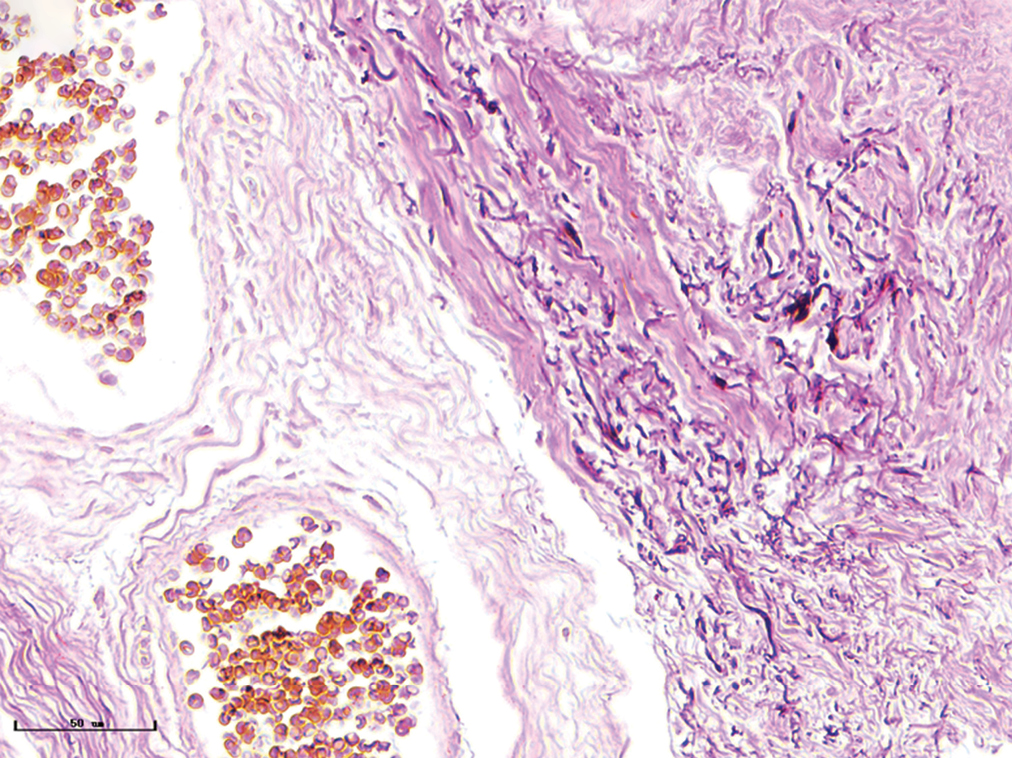
The changes of collagen and elastic fibres in normal skin and different types of port-wine stains were shown in Figures 4 and 5. The collagen fibres in red and purple port-wine stains were arranged neatly and uniformly, and stained evenly and densely, as in normal skin. In hypertrophic and nodular port-wine stains, a large number of red blood cells were visible within the vessels. Loose collagen fibres that were thinner than those of normal skin, were visible around the vessels. In nodular port-wine stains, vascular smooth muscle, staining red with Masson’s trichrome staining, and confirmed with immunofluoresce, indicated that arterial or venous malformations were involved in the development of port-wine stains. The elastic fibres in red port-wine stains and purple port-wine stains were arranged neatly and uniformly, dot-like and line-like patterns, consistent with normal skin. In hypertrophic and nodular port-wine stains, the number of elastic fibres were greater and in a disorderly arrangement. Multi-layered elastic fibres with a wavy arrangement in the vessels were observed in nodular port-wine stains. Combined with the results of immunofluorescent and Masson’s staining, the port-wine stains lesions appeared to be composed mainly of capillary and venous malformations. Arteriovenous malformations were apparent in the later stages of port-wine stains.
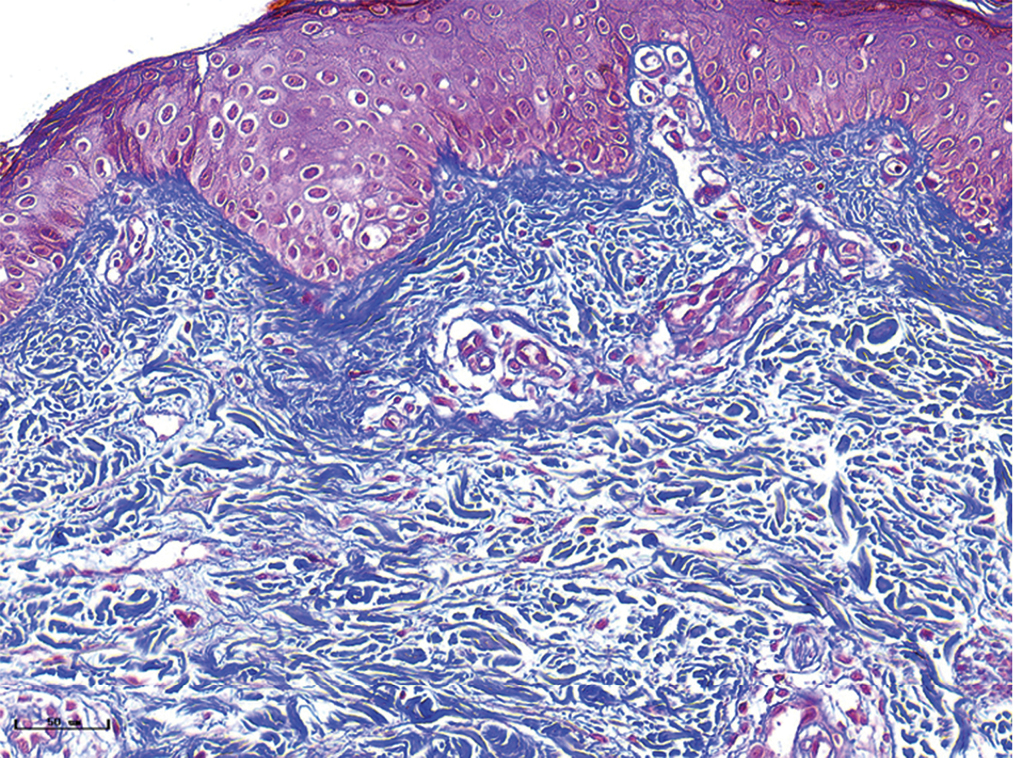
- Normal skin, the collagen fibres were blue and the vascular smooth muscles were red. collagen fibres were arranged neatly and uniformly (Masson’s trichrome staining, ×400)

- Red port-wine stains, dilated vessels were observed, and the collagen fibres were arranged neatly and uniformly (Masson’s trichrome staining, ×400)
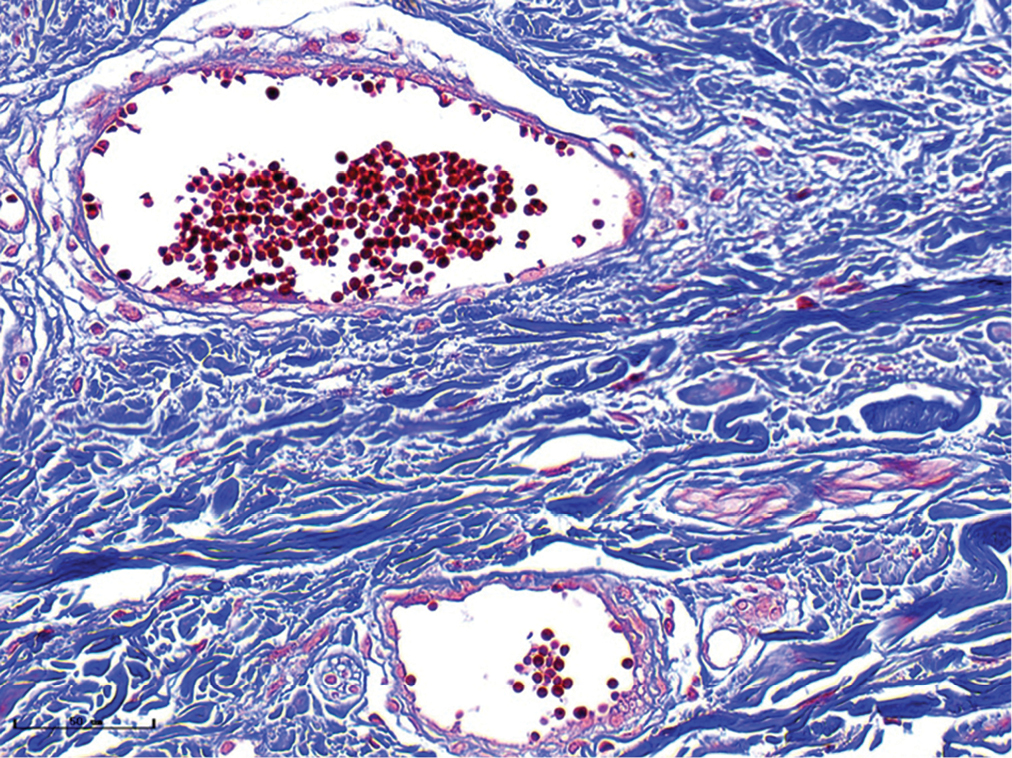
- Purple port-wine stains, vessels were obviously dilated, and the collagen fibres were arranged neatly and uniformly (Masson’s trichrome staining, ×400)
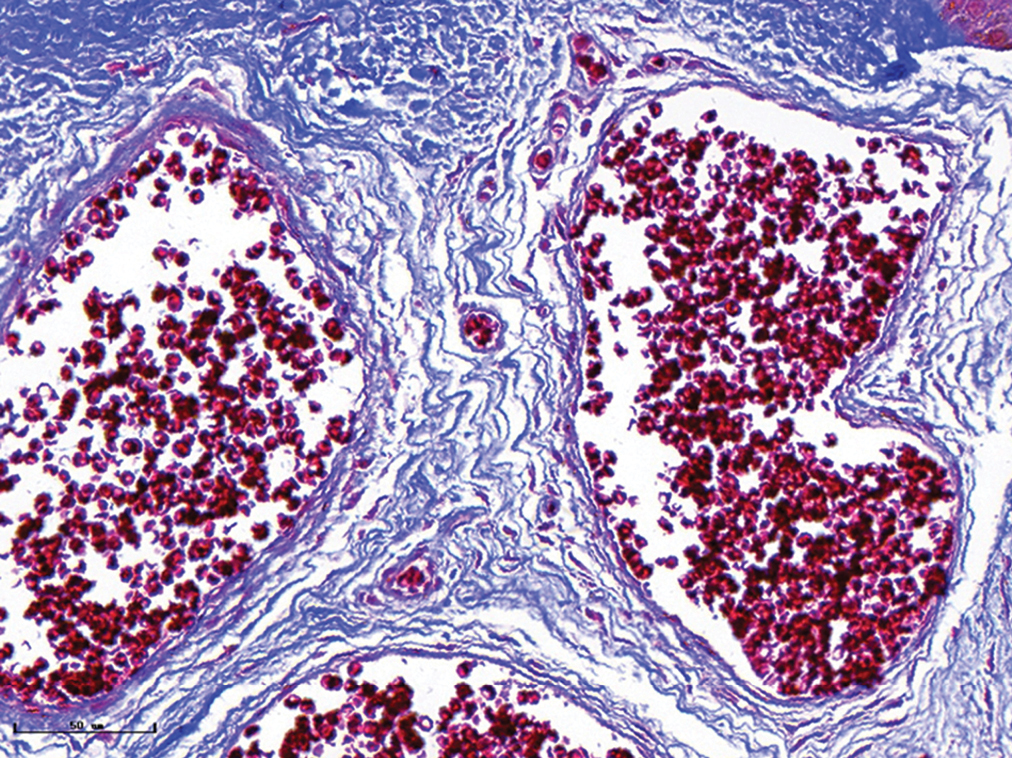
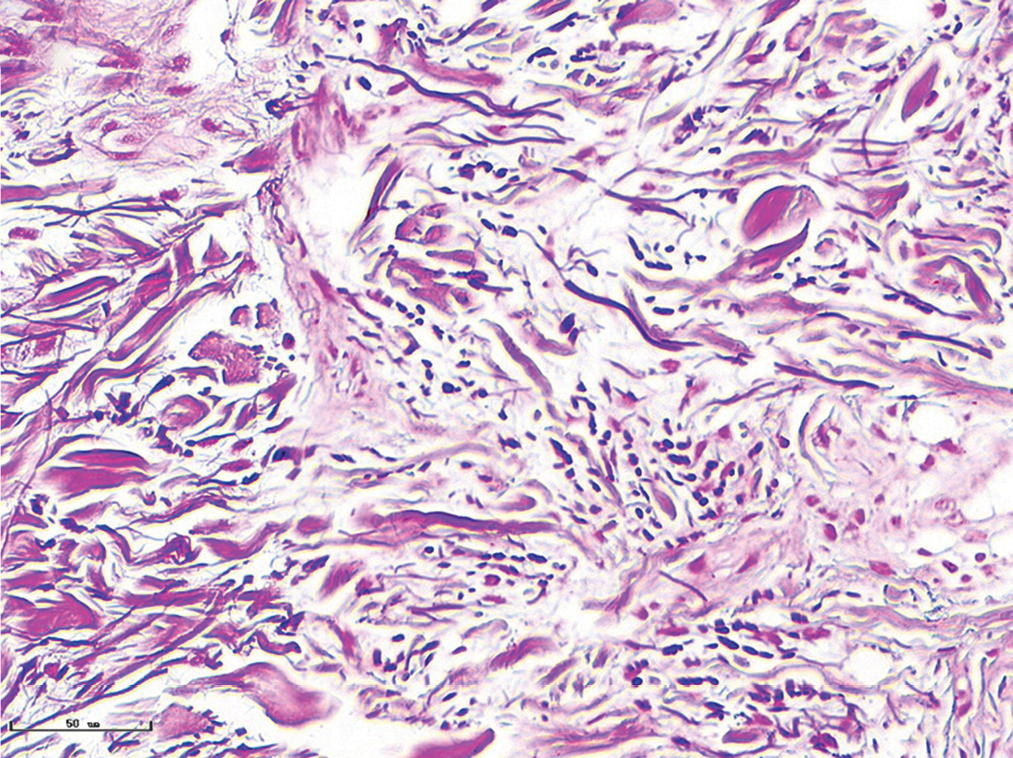
- Hypertrophic port-wine stains, vessels were significantly dilated, and collagen fibres were arranged loosely and thinly (Masson’s trichrome staining, ×400)
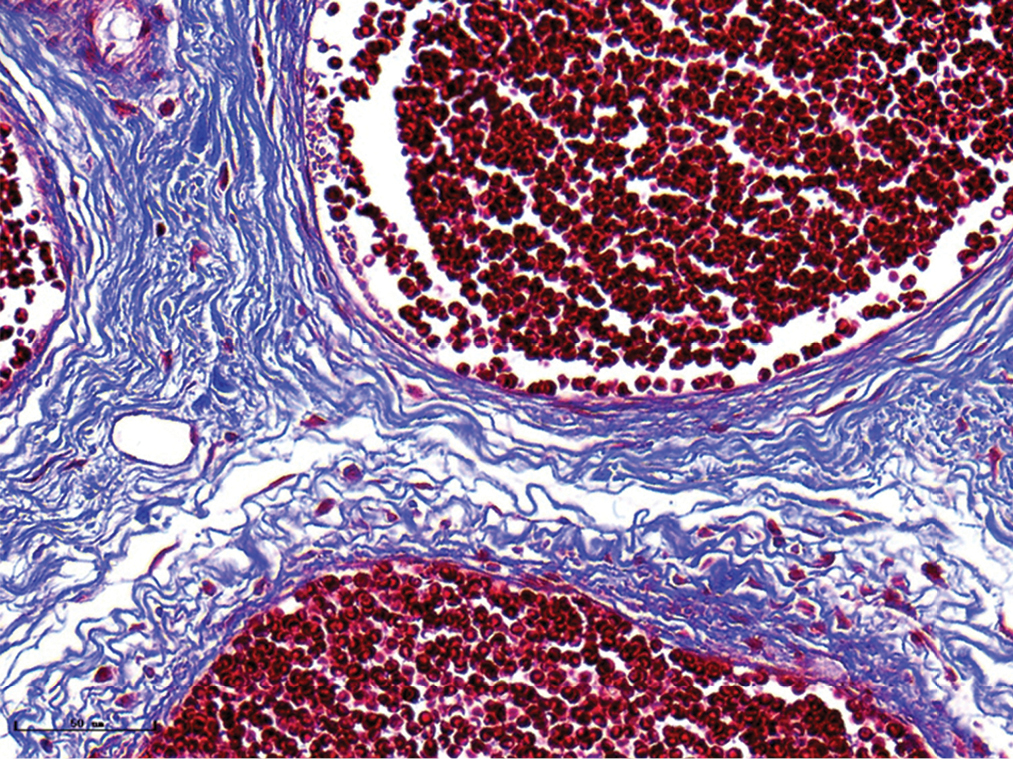
- Nodular port-wine stains, vessels were significantly dilated, and collagen fibers were arranged loosely and thinly (Masson’s trichrome staining, ×400)
- Vascular smooth muscles (black arrow) were observed in nodular port-wine stains (Masson’s trichrome staining, ×400)
- Normal subject, elastic fibres formed in linear and dotted pattern (Weigert staining, ×400)
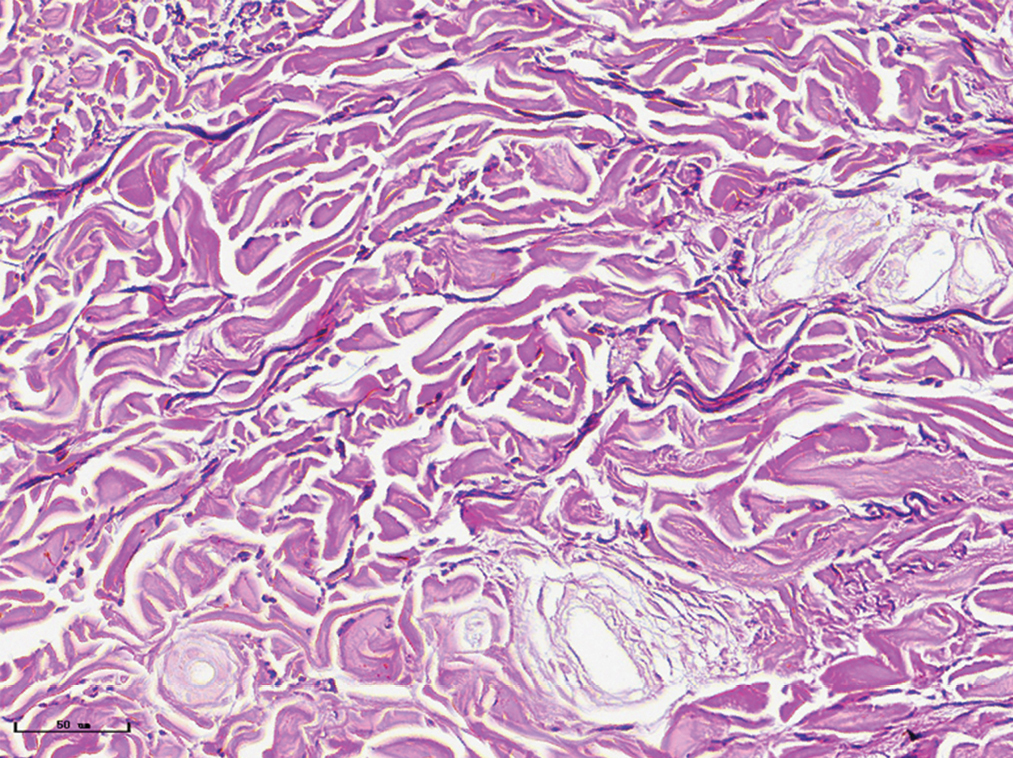
- Red port-wine stains, elastic fibres were arranged neatly (Weigert staining, ×400)
- Purple port-wine stains, elastic fibres were increased and distributed in dotted pattern (Weigert staining, ×400)
- Hypertrophic port-wine stains, elastic fibres were thickened and arranged disorderly (Weigert staining, ×400)
- Nodular port-wine stains, elastic fibers were thickened and arranged disorderly (Weigert staining, ×400)
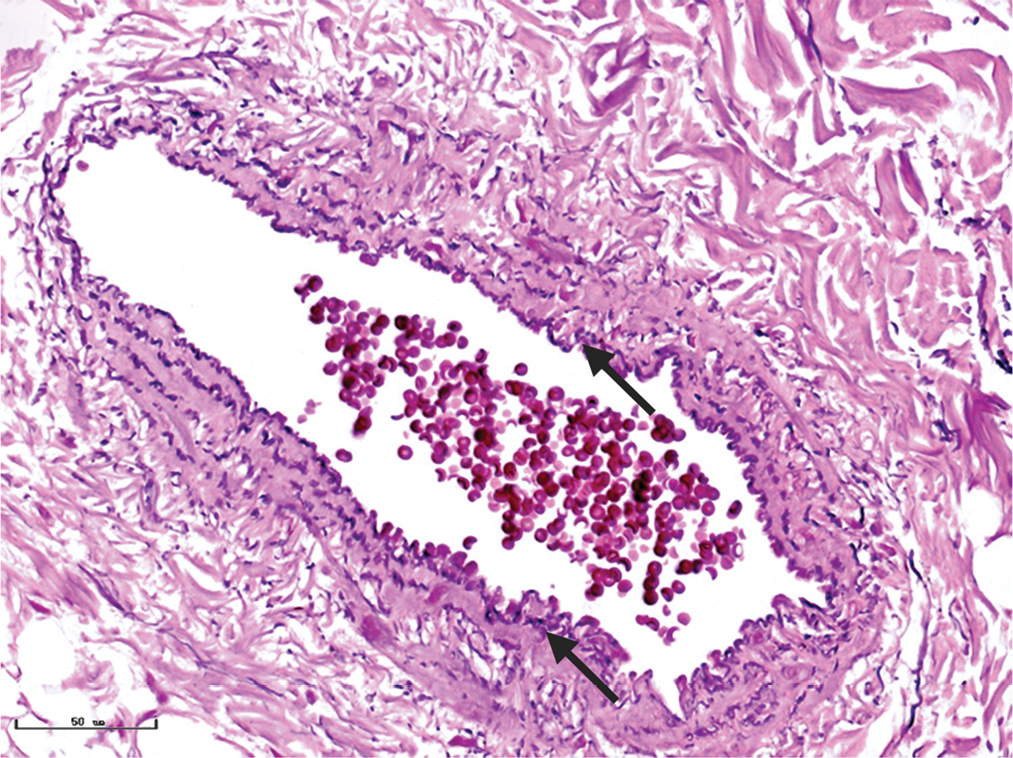
- Multiple layers of elastic fibres (black arrows) in a vessel formed in a wavy pattern in nodular port-wine stains (Weigert staining, ×400)
Discussion
Fiskerstrand et al.13 measured the depth of vessels in port-wine stains using full-length biopsies, finding a mean vessel depth of 240 ± 80 μm. Meanwhile, the maximum depth of the vessels was 800 μm. Optical coherence tomography was a noninvasive technique that provided a penetration depth of 1.5 mm. One study revealed that the mean vessel depth was 304 ± 99 μm by Doppler optical coherence tomography.14 Another study by Liu et al.15 demonstrated that dilated vessels were located within the region 600–1,000 μm below the surface of the skin using Doppler optical coherence tomography. In the present study, we found that the mean vessel depth was 944.2 ± 105.4 μm and their maximum depth was 1,369.3 μm in purple port-wine stains. The mean vessel depth was 2,971 ± 161.3 μm and their maximum depth was 3,474.3 μm in hypertrophic port-wine stains. These results were greater than those recorded in previous studies. The possible reason for this discrepancy may be related to the limited penetration depth of optical coherence tomography. Therefore, histopathological examination remains an important method of evaluation of the vascular characteristics of port-wine stains.
In a prospective study, Qiu et al.16 used a customised diffuse reflectance spectroscopy probe to evaluate the features of different types of port-wine stains (pink: flat; red: flat; purple: slightly thicker; thickened purple: significantly thicker). From pink to thickened purple, mean vessel diameter was found to be 28.24 ± 10.59 μm, 34.18 ± 11.21 μm, 68.74 ± 28.10 μm and 96.94 ± 31.36 μm, respectively. A different study demonstrated that the mean vessel diameter in port-wine stains was 114 ± 92 μm.14 Angiographic optical coherence tomography imaging of port-wine stains exhibited heterogeneous vessel patterns with vessel diameters ranging from 20 to 160 μm.17 Zhou et al.18 revealed that the mean vessel diameter of purple and proliferative port-wine stains was 125.63 ±19.09 μm and 193.93 ± 32.43 μm, respectively, when measured by optical coherence tomography. The mean vessel diameter of purple and hypertrophic port-wine stains was 93.5 ± 9.7 μm and 155.6 ± 21.8 μm, respectively, in our study, similar to previous findings by optical coherence tomography. Taken together, these observations suggest that optical coherence tomography may be considered as useful for measurement of the mean vessel diameter in port-wine stains. However, the limited penetration depth of optical coherence tomography influenced the accuracy of those measurements. Although pathological biopsies objectively show changes in the blood vessels in skin lesions and provide accurate data for treatment, it isn’t easy to do, being an invasive test. Therefore, non-invasive techniques for evaluating port-wine stains should be further evaluated.
Until now, pulsed-dye laser remains the gold standard for the treatment of port-wine stains. In order to understand its therapeutic properties, researchers have conducted histopathological analysis of port-wine stains before and after pulsed-dye laser treatment. They found that coagulated RBCs and vessel wall coagulation were limited to a maximum depth of 1.3 mm (0.86 ± 0.32 mm) and 0.65 mm (0.37 ± 0.17 mm), respectively.19 Meanwhile, vessels with a diameter greater than 200 μm, only RBCs in the upper half (towards the direction of incident light) showed the greatest thermal damage, while red blood cells in the lower half demonstrated only slight damage.19 In another study, pulsed-dye laser demonstrated good efficacy for vessels to a depth of less than 400 μm in the dermis with mean vessel diameter of 38 ± 19 μm, but poor efficacy for vessels with mean vessel diameter of 19 ± 6.5 μm.20 In the present study, the mean vessel diameter and vessel depth for red port-wine stains were 38.7 ± 5.9 μm and 396.4 ± 31 μm, respectively, consistent with the best treatment results with pulsed-dye laser. Therefore, the potential curative effect in red port-wine stains was better than that of other types. In addition, as the disease progresses, the diameter and depth of vessels in purple, hypertrophic, and nodular port-wine stains gradually increases (diameter: purple port-wine stains: 93.5 ± 9.7 μm, hypertrophic port-wine stains: 155.6 ± 21.8 μm, nodular port-wine stains: 155.6 ± 29.54 μm; depth: purple port-wine stains: 944.2 ± 105.4 μm, hypertrophic port-wine stains: 2,971 ± 161.3 μm, nodular port-wine stains: 3,594 ± 364.6 μm). The above results suggested that the diameter and depth of vessels of these types of port-wine stains were greater than the best treatment parameters that pulsed-dye laser could offer.
The 755 nm-Alexandrite laser can penetrate 50%~75% deeper into the skin than pulsed-dye laser, with less epidermal melanin absorption.21 A previous study has revealed that Alexandrite laser was effective for the treatment of resistant port-wine stains, although the therapeutical window was narrow.22 Moreover, the penetration depth of 1064 nm Nd:YAG laser is 4–7 mm into the dermis.23 Hence, a Long-pulsed 1064 nm Nd:YAG laser has been used to treat hypertrophic port-wine stains, which was highly effective when used for a small number of treatment sessions.24 However, mild side effects were observed in half of the patients. Recently, photodynamic therapy (PDT) has been used as a treatment for port-wine stains with promising results in China. Han et al.25 conducted a retrospective study of PDT for pulsed-dye laser-resistant port-wine stains. It revealed that 46.2% of patients displayed excellent or good levels of improvement (>50% colour blanching) with adverse events that were minimal and self-limiting. Therefore, the various treatment methods described above can be used to treat pulsed-dye laserresistant port-wine stains in addition to purple and hypertrophic port-wine stains. Nodular port-wine stains could be treated with a CO2 laser or 1064 nm Nd:YAG laser.26
Tan et al.12 described pathological alterations in infantile and early childhood port-wine stains (red port-wine stains). They found that the number of layers of pericytes and basement membranes clearly increased, resulting in thickened vessel walls. Transmission electron microscopy indicated a normal capillary venular structure of the superficial plexus. With disease progression, several layers of fluorescence for αSMA were observed, indicating that in addition to malformations in capillaries, there were also those of veins and arteries. In addition, we performed elastic fibre staining on port-wine stains, finding that there were multiple layers of elastic fibres and abundant muscle fibres in nodular port-wine stains, further confirming our conjecture that arteries might appear in the later stages of port-wine stains. A total of 31 cases of nodular port-wine stains were analysed by Chen et al.27 who found that those cases were purulent granulomas, arteriovenous malformations, cavernous vasodilation. Our study found that in nodular port-wine stains, two patients had arteriovenous malformations, one had cavernous vasodilation, and the other cases had venous malformations. Vessels in other types of port-wine stains mainly exhibited capillary and venous malformations. The research results described above demonstrate that as the disease progresses, the vascular composition of port-wine stains changes, the diameter of vessels, their depth and the difficulty of treating increases. This also explains the difference in efficacy of treatment of different types of port-wine stains.
Limitation
The lesions were predominantly located on the face, so it was difficult to obtain samples. Hence, a small number of patients were included in the present study.
Conclusion
In conclusion, our study explored vessel diameter and depth in different types of port-wine stains, which not only explained the possible reasons for the resistance to pulsed-dye laser treatment but also provided a scientific basis for treatment options. Our study also dynamically showed the histological changes from red port-wine stains to nodular port-wine stains which suggested that the difficulty of treatment increases as the disease progresses.
Declaration of patient consent
Institutional Review Board (IRB) permission was obtained for the study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
This study was supported by grants from National Natural Science Foundation of China (Grant No. 82003373 and 82073473), Department of Science and Technology of Sichuan Province (Grant No. 2021YJ0426), Post-Doctor Research Project and the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University(Grant No.2020HXBH058 and ZYJC21036).
References
- Safety and efficacy of dual-wavelength laser (1064 + 595 nm) for treatment of non-treated port-wine stains. J Eur Acad Dermatol Venereol. 2018;32:260-4.
- [CrossRef] [PubMed] [Google Scholar]
- Simulating light transport through skin for color prediction of port wine stain lesions: A review. J Biomed Opt. 2012;17:110901.
- [CrossRef] [PubMed] [Google Scholar]
- Port-wine stains unresponsive to pulsed dye laser: Explanations and solutions. Br J Dermatol. 1998;139:173-7.
- [CrossRef] [PubMed] [Google Scholar]
- Port-wine stains. An assessment of 5 years of treatment. Arch Otolaryngol Head Neck Surg. 1996;122:1174-9.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrence of port-wine stains after treatment with the flashlamp-pumped pulsed dye laser. Br J Dermatol. 2000;143:1230-4.
- [CrossRef] [PubMed] [Google Scholar]
- Port wine stain treatment outcomes have not improved over the past three decades. J Eur Acad Dermatol Venereol. 2019;33:1369-77.
- [CrossRef] [PubMed] [Google Scholar]
- Pulsed dye laser-resistant port-wine stains: mechanisms of resistance and implications for treatment. Br J Dermatol. 2013;168:941-53.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of vascular skin lesions with the variable-pulse 595 nm pulsed dye laser. Dermatol Surg. 2006;32:41-8.
- [CrossRef] [PubMed] [Google Scholar]
- Demographic study of port wine stain patients attending a laser clinic: Family history, prevalence of naevus anaemicus and results of prior treatment. Clin Exp Dermatol. 1997;22:166-8.
- [CrossRef] [PubMed] [Google Scholar]
- Are arteriovenous malformations a causative factor for hypertrophic and nodular port-wine stains? J Am Acad Dermatol. 2014;71:e219-21.
- [CrossRef] [PubMed] [Google Scholar]
- Hemoporfin photodynamic therapy for port-wine stain: A randomized controlled trial. PloS One. 2016;11:e0156219.
- [CrossRef] [PubMed] [Google Scholar]
- Pathological alterations involve the entire skin physiological milieu in infantile and early childhood port wine stain. Br J Dermatol. 2016;177:293-6.
- [CrossRef] [PubMed] [Google Scholar]
- Laser treatment of port wine stains: Therapeutic outcome in relation to morphological parameters. Br J Dermatol. 1996;134:1039-43.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of skin port-wine stain and hemangioma vascular lesions using Doppler OCT. Skin Res Technol. 2016;22:223-29.
- [CrossRef] [PubMed] [Google Scholar]
- In vivo, high-resolution, three-dimensional imaging of port wine stain microvasculature in human skin. Lasers Surg Med. 2013;45:628-32.
- [CrossRef] [PubMed] [Google Scholar]
- Determination of optical and microvascular parameters of port wine stains using diffuse reflectance spectroscopy. Adv Exp Med Biol. 2016;923:359-65.
- [CrossRef] [PubMed] [Google Scholar]
- Angiographic optical coherence tomography imaging of hemangiomas and port wine birthmarks. Lasers Surg Med 2018
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of skin microvessels with optical coherence tomography: potential uses in port wine stains. Exp Ther Med. 2012;4:1017-21.
- [CrossRef] [PubMed] [Google Scholar]
- Epidermal damage and limited coagulation depth with the flashlamp-pumped pulsed dye laser: A histochemical study. J Invest Dermatol. 1995;104:798-802.
- [CrossRef] [PubMed] [Google Scholar]
- Photothermally induced vessel-wall necrosis after pulsed dye laser treatment: lack of response in port-wine stains with small sized or deeply located vessels. J Invest Dermatol. 1996;107:671-5.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment endpoints for resistant port wine stains with a 755 nm laser. J Cosmet Laser Ther. 2009;11:52-5.
- [CrossRef] [PubMed] [Google Scholar]
- Alexandrite laser for the treatment of resistant and hypertrophic port wine stains: A clinical, histological and histochemical study. Actas dermo-sifiliograficas. 2016;107:591-6.
- [CrossRef] [PubMed] [Google Scholar]
- Classification and treatment of orbital venous malformations: an updated review. Front Med. 2019;13:547-55.
- [CrossRef] [PubMed] [Google Scholar]
- Long-pulsed 1064 nm Nd:YAG laser improves hypertrophic port-wine stains. J Eur Acad Dermatol Venereol. 2013;27:1381-6.
- [CrossRef] [PubMed] [Google Scholar]
- Retrospective study of photodynamic therapy for pulsed dye laser-resistant port-wine stains. J Dermatol. 2020;47:348-55.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of hypertrophic and nodular port-wine stains with intralesional 1064 nm Nd:YAG laser. Dermatol Ther. 2020;33:e13925.
- [CrossRef] [Google Scholar]
- Nodules arising within port-wine stains: a clinicopathologic study of 31 cases. Am J Dermatopathol. 2011;33:144-51.
- [CrossRef] [PubMed] [Google Scholar]






