Translate this page into:
Plants and microbes: Source of dermatology drugs
Corresponding author: Dr. Budeda Hasini, 16a-5-1, Himaja Hospital, Tangellamudi, East Godavari, Andhra Pradesh, India. surupgsa@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Purushothaman S, Hasini B, Mayakuntla B, Rudrapriya S, Sudevan D, Ravi SS, et al. Plants and microbes: Source of dermatology drugs. Indian J Dermatol Venereol Leprol 2023;89:487-92.
Since time immemorial, natural products have been the backbone of traditional system of healing throughout the globe and have also been an integral part of history and culture. It has been well documented that natural products played critical roles in modern drug development, especially for antibacterial and antitumor agents. Until recently, plants were an important source of novel pharmacologically active compounds with many drugs being derived directly or indirectly from plants. Despite the current preoccupation with synthetic chemistry as a vehicle to discover and manufacture drugs, the contribution of plants to disease treatment and prevention is still enormous. In this article, we enumerate the structure of drugs and its derivative.1
Systemic Antimicrobials
Penicillin G
It is derived from a fungus Penicillium chrysogenum2 [Figure 1a] having broad-spectrum antibacterial action which has a five-membered thiazolidine ring [Figure 1b].3

- Penicillium chrysogenum
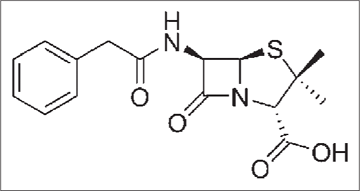
- Structure of penicillin
Cephalosporins
It is derived from a mould Cephalosporium acremonium. It has a four-membered β-lactam ring attached to a sixmembered dihydrothiazine ring and, therefore, belongs to β-lactams.2
Vancomycin
A macrolide derived from actinomycetes Streptomyces orientalis which is a glycopeptide antibiotic used in the treatment of staphylococcal infections that are resistant to conventional antibiotics.2
Rifamycin
It is derived from a soil mould Amycolatopsis rifamycinica/ Streptomyces mediterranei.
Clindamycin
It is derived from Streptomyces lincolnensis. The drug is a derivative of lincomycin that has increased antibacterial activity and is better absorbed than its parent drug.2
Erythromycin
It is derived from Saccharopolyspora erythraea. It contains macrocyclic lactone rings. It has specific anti-inflammatory properties, contributes to their therapeutic benefit in inflammatory facial dermatoses such as acne and rosacea.2
Tetracycline
It is derived from Streptomyces species It has four fused six-membered rings and is bacteriostatic with greater gram-positive than gram-negative activity.2
Systemic Antifungals
Griseofulvin
It is derived from a mould Penicillium griseofulvum which is indicated for the treatment of dermatophyte infections of the skin, scalp and nails.4
Amphotericin B
It is derived from Streptomyces nodosus used in the treatment of systemic fungal infections and leishmaniasis.4
Systemic Antiparasitic
Ivermectin
It is derived from Streptomyces avermitilis [Figure 2a].5 It is the 22, 23-dihydro derivative of avermectin [Figure 2b] B1 and is classified as a macrocyclic in the avermectin family.6

- Streptomyces avermitilis
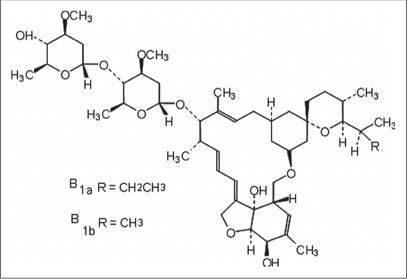
- Structure of ivermectin
Topicals
Bacitracin
It is derived from Licheniformis group of Tracy 1 strain of Bacillus subtilis. Bacitracin complexes with the carrier protein C55-prenol pyrophosphatase which is involved in bacterial cell wall synthesis.7
Polymyxin B
Polymyxin B is a cationic branched cyclic decapeptide isolated from the aerobic gram-positive rod Licheniformis group of Tracy 1 strain of Bacillus subtilis.7
Neomycin
Neomycin is a bactericidal aminoglycoside antibacterial agent produced by Streptomyces fradiae.7
Mupirocin
It inhibits bacterial isoleucyl-tRNA synthetase, thereby hindering bacterial RNA, protein and cell wall synthesis derived from Pseudomonas fluorescens.7
Retapamulin
Retapamulin is a semi-synthetic pleuromutilin derivative and the first topical antibacterial in the pleuromutilin class developed for human use. Pleuromutilin is a tricyclic diterpene produced by Clitopilus scyphoides (previously known as Pleurotus mutilus).7
Gentamicin
It is derived from Micromonospora purpurea. Enclosure of gentamicin-collagen sponge following primary excision in hidradenitis suppurativa, has reduced the rate of complications one week postoperatively without affecting the recurrence rates.7
Nystatin
It is derived from Streptomyces noursei used as an antifungal agent.8
Permethrin
Pyrethrins are organic compounds originally derived from a flower species of the genus Compositae, which is related to the chrysanthemum used in scabies and ticks.9
Immunosuppressants
Mycophenolic acid
It is derived from Penicillium brevicompactum [Figure 3a].[10] It has antibacterial, antiviral, antifungal, antitumour and immunosuppressive properties. It is the morpholinoethyl ester of mycophenolic acid [Figure 3b].11

- Pencillium brevicompactum
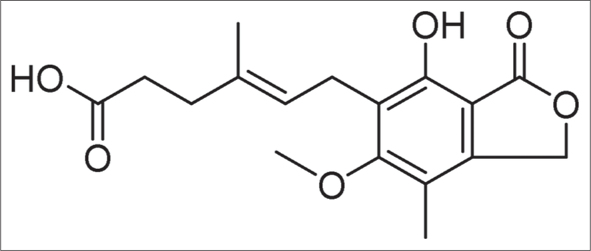
- Structure of mycophenolic acid
Cyclosporine
It is derived from soil fungus, Tolypocladium inflatum gams12 [Figure 4a] which is a neutral cyclic peptide composed of 11 amino acids [Figure 4b].13

- Tolypocladium inflatum gams
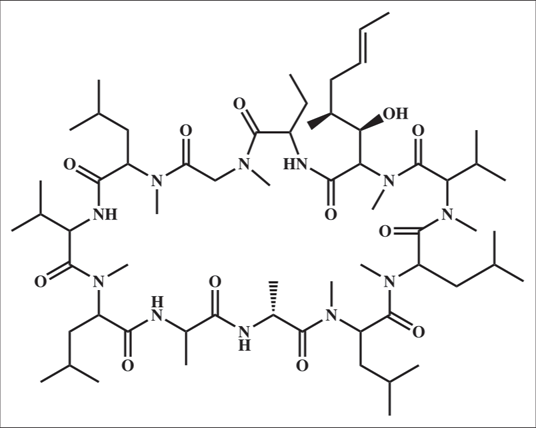
- Structure of cyclosporine
Tacrolimus
It is derived from Streptomyces tsukubaensis. Tacrolimus is a macrolide that inhibits calcineurin by binding to the FK506 binding protein.14
Pimecrolimus
It is an immunomodulating agent of the calcineurin inhibitor15 class which is a epi- chloro- derivative of the asxomycin [Figure 5a] used in the treatment of atopic dermatitis derived from Streptomyces hygroscopicus [Figure 5b].16
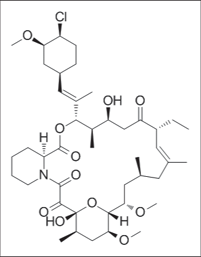
- Structure of pimecrolimus
- Streptomyces hydroscopicus
Chemical Peels
Glycolic acid
The smallest of the alpha-hydroxy acid is the two-carbon [Figure 6]17 molecule, glycolic acid. It contains the formula HOCH2COOH derived from sugarcane.18
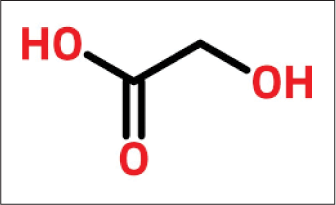
- Structure of glycolic acid
Lactic acid
Lactic acid can exist in several isomeric forms: L-lactic acid19,20 (levorotatory), the D-lactic acid (dextrorotatory) [Figure 7] or the DL racemic mixture which is derived from sour milk, bilberries and yogurt.
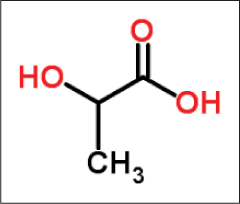
- Structure of lactic acid
Malic acid
Malic acid was first described by Scheele in 1785, who isolated this acid from unripe apples. It is found in other fruits such as grapes, watermelons and cherries and in vegetables such as carrots and broccoli. It is dicarboxylic acid [Figure 8].21
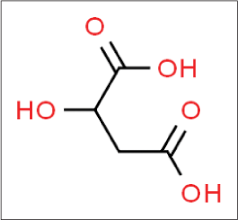
- Structure of malic acid
Mandelic acid
Mandelic acid is named after the German word ‘mandel’22 for almond, as it is derived from bitter almond extract. It is an eight-carbon alpha-hydroxy acid (molecular formula C6H5CH[OH]COOH) [Figure 9],23 and it is also available in two enantiomeric and pharmacologically distinct forms.
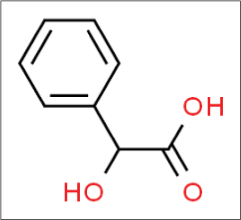
- Structure of mandelic acid
Tartaric acid
Tartaric acid is an abundant constituent24 of many fruits such as grapes and bananas and exhibits a slightly astringent and refreshing sour taste. It is carboxylic acid with molecular formula C4H6O6 [Figure 10].25
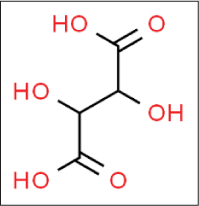
- Structure of tartaric acid
Ferulic acid
It is a phenolic compound and an effective scavenger of free radicals derived from cereals.
Phytic acid
Phytic acid is only found in plant-derived foods. All edible seeds, grains, legumes and nuts contain in varying quantities, and small amounts are also found in roots and tubers.26
Arginine
It is a large molecule and hence does not cause any irritation to the skin. It is derived from brown sugar. It is an alpha-amino acid containing a 3-carbon aliphatic straight chain ending in a guanidine group [Figure 11].27
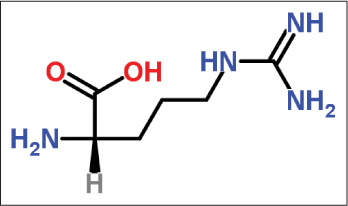
- Structure of arginine
Black peel
It is derived from fermentation of black rice.
Jasmonic acid
It is derived from jasmine.28
Miscellaneous
Kojic acid
It is derived from the fungus Aspergillus oryzae [Figure 12a], used as a depigmenting agent. It is a pyranone that is 4H - pyran substituted by a hydroxyl group 5, a hydroxymethyl group at position 2 and oxo group at position 4 [Figure 12b].29

- Aspergillus oryzae
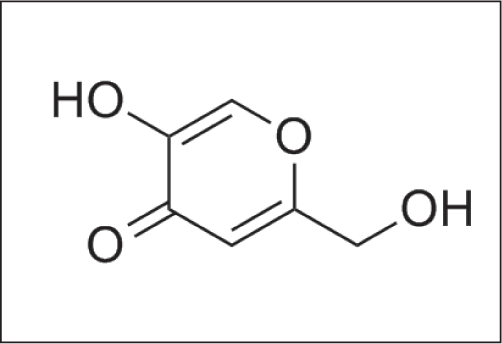
- Structure of kojic acid
Podophyllin
It is derived from the roots of the mayapple (Podophyllin peltatum). It is cytotoxic agent used in the treatment of external genital warts and condyloma acuminatum.30
Sinecatechins
It is derived from green tea polyphenol extract from Camellia sinensis used in the treatment of external genital and perianal warts.31
Colchicine
It is derived from Colchicum autumnale [Figure 13a] used as an anti-inflammatory agent for treatment of Behcet disease.32 It ia an alkaloid having molecular formula C22H25NO6 [Figure 13b].

- Colchicum autumnale
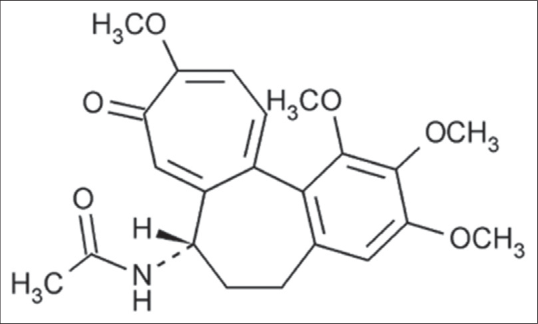
- Structure of colchicine
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Natural products derived from plants as a source of drugs. J Adv Pharm Tech Res. 2012;3:200-1.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic antibacterial agents In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy (3th ed). Amsterdam, Netherlands: Elsevier; 2013. p. :61-97.
- [CrossRef] [Google Scholar]
- Kinetic study of antibiotic by reverse micelle extraction technique. J Taiwan Inst Chem Eng. 2012;43:685-95.
- [CrossRef] [Google Scholar]
- Systemic antifungal drugs In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy, Part 3 (3th ed). Amsterdam, Netherlands: Elsevier; 2013. p. :98-119.
- [CrossRef] [Google Scholar]
- Systemic antiparasitic drugs In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy, Part 3 (3th ed). Amsterdam, Netherlands: Elsevier; 2013. p. :135-7.
- [CrossRef] [Google Scholar]
- Pharmacokinetic profile of ivermectin in cattle dung excretion, and its associated environmental hazard. Soil Sediment Contam. 2009;18:564-75.
- [CrossRef] [Google Scholar]
- Topical antibacterial agents In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy, Part 8 (3th ed). Amsterdam, Netherlands: Elsevier; 2013. p. :445-59.
- [CrossRef] [Google Scholar]
- Topical antifungal drugs In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy, Part 7 (83th ed). Amsterdam, Netherlands: Elsevier; 2013. p. :460-2.
- [CrossRef] [PubMed] [Google Scholar]
- Topical antiparasitic agents In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy, Part 8 (3th ed). Amsterdam, Netherlands: Elsevier; 2013. p. :481-3.
- [CrossRef] [Google Scholar]
- Mycophenil mofetil In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy, Part 4 (3th ed). Amsterdam, Netherlands: Elsevier; 2013. p. :190-1.
- [CrossRef] [Google Scholar]
- Emerging mycotoxins: Beyond traditionally determined food contaminants. J Agric Food Chem. 2016;65:10.
- [CrossRef] [PubMed] [Google Scholar]
- Cyclosporine In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy, Part 4 (3th ed). Amsterdam, Netherlands: Elsevier; 2013. p. :199-203.
- [CrossRef] [Google Scholar]
- Optimization of a solid-phase extraction procedure for the analysis of drug-loaded lipid nanoparticles and its application to the determination of leakage and release profiles. J Pharm Sci. 2020;109:10.
- [CrossRef] [PubMed] [Google Scholar]
- Cytotoxic and antimetabolic agents In: Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ, eds. Fitzpatrick's Dermatology, Part 28 (9th ed). United States: McGraw-Hill Inc; 2019. p. :3463-77.
- [Google Scholar]
- PubChem Compound Summary for CID 6509979, Pimecrolimus Bethesda, Maryland, US: National Center for Biotechnology Information; 2021.
- [Google Scholar]
- Topical calcineurin inhibitors In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy, Part 8 (3th ed). Amsterdam, Netherlands: Elsevier; 2013. p. :531-45.
- [Google Scholar]
- PubChem Compound Summary for CID 757, Glycolic Acid Bethesda, Maryland, US: National Center for Biotechnology Information; 2021.
- [Google Scholar]
- The Therapeutic value of glycolic acid peels in dermatology. Indian J Dermatol Venereol Leprol. 2020;69:148-50.
- [PubMed] [Google Scholar]
- Newer superficial peels In: ACS(1) Cutaneous and Aesthetic Surgery. Ch. 40B. Mysore: Venkataraman; p. :592-603.
- [CrossRef] [Google Scholar]
- PubChem Compound Summary for CID 612, Lactic Acid Bethesda, Maryland, US: National Center for Biotechnology Information; 2021.
- [Google Scholar]
- PubChem Compound Summary for CID 525, Malic Acid Bethesda, Maryland, US: National Center for Biotechnology Information; 2021.
- [Google Scholar]
- Organic and Fatty Acid Production, Microbial the Hebrew University of Jerusalem, Jerusalem, Israel. Amsterdam, Netherlands: Elsevier Inc.; 2009. p. :421-42.
- [CrossRef] [Google Scholar]
- PubChem Compound Summary for CID 1292 Mandelic Acid Bethesda, Maryland, US: National Center for Biotechnology Information; 2021.
- [Google Scholar]
- Chemical peels, 541 In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy, Part 10 (3th ed). Amsterdam, Netherlands: Elsevier; 2013. p. :579-82.
- [CrossRef] [Google Scholar]
- PubChem Compound Summary for CID 875, Tartaric Acid Bethesda, Maryland, US: National Center for Biotechnology Information; 2021.
- [Google Scholar]
- Antioxidant functions of phytic acid. Free Radic Biol Med. 1990;8:61-9.
- [CrossRef] [PubMed] [Google Scholar]
- PubChem Compound Summary for CID 6322 Arginine Bethesda, Maryland, US: National Center for Biotechnology Information; 2021.
- [Google Scholar]
- Jasmonates and tetrahydrojasmonic acid: A novel class of anti-aging molecules. J Drugs Dermatol. 2016;15:206-7.
- [PubMed] [Google Scholar]
- PubChem Compound Summary for CID 3840 Kojic Acid Bethesda, Maryland, US: National Center for Biotechnology Information; 2021.
- [Google Scholar]
- Podophyllin and its use in the treatment of condylomata acuminata. Indian J Dermatol Venereol Leprol. 1990;56:10-4.
- [Google Scholar]
- Sinecatechins ointment, 15% for the treatment of external genital and perianal warts: Proceedings of an expert panel roundtable meeting. J Clin Aesthet Dermatol. 2016;9(Suppl 3):S2-15.
- [Google Scholar]
- A simple (HPLC-UV) method for the quantification of colchicine in bulk and ethosomal gel nano-formulation and its validation. Int J Pharm Pharm Sci. 2017;9:72-8.
- [CrossRef] [Google Scholar]





