Translate this page into:
Intralesional immunotherapy for non-genital warts: A systematic review and meta-analysis
Corresponding Author: Dr. Ji Hae Lee, Department of Dermatology, St. Vincent’s Hospital, Paldal-gu, Suwon, South Korea. l.jihaemd@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ju HJ, Park HR, Kim JY, Kim GM, Bae JM, Lee JH. Intralesional immunotherapy for non-genital warts: A systematic review and meta-analysis. Indian J Dermatol Venereol Leprol 2022;88:724-37.
Abstract
Background
Intralesional immunotherapy has been reported to be effective for warts and to show good safety profiles, but this has not yet been systematically studied.
Aims
To determine the efficacy and safety of intralesional immunotherapy for treating non-genital warts.
Methods
We comprehensively searched the MEDLINE, Embase, Web of Science and Cochrane Library databases from the times of their inception to January 3, 2020. The primary outcome was the rate of complete response of all lesions. The distant complete response rate of warts located in an anatomically different body part and the recurrence rate were also analyzed.
Results
A total of 54 prospective studies was ultimately included. The immunotherapeutic agents used were Mycobacterium w vaccine, measles, mumps and rubella vaccine, purified protein derivative, Candida antigen, interferon, bacillus Calmette-Guérin vaccine and others. The pooled rate of complete response among all patients with non-genital warts treated using intralesional immunotherapy was 60.6% (95% confidence interval 54.8–66.5%). The pooled recurrence rate was 2.0% (95% confidence interval, 1.1–2.9%). All reported adverse events were mild and transient.
Limitations
The heterogeneity among studies
Conclusion
Intralesional immunotherapy is suggested for use in patients with multiple warts, given its promising results, good safety profile and low recurrence rate.
Keywords
Intralesional immunotherapy
immunotherapy
non-genital wart
systematic review
warts
Plain Language Summary
Intralesional immunotherapy has been reported to be effective for warts, but this has not been systematically studied. In this meta-analysis, intralesional immunotherapy demonstrated significant therapeutic effects on non-genital warts with high safety profiles and low recurrence rates and can be recommended for use in patients with multiple non-genital warts.
Introduction
Warts are the most common clinical manifestation of human papillomavirus on the skin and mucous membranes. They can greatly affect a patient’s quality of life by causing embarrassment, fear of negative appraisal by others, and frustration due to persistent recurrence.1,2 Various treatment methods are available, such as physical destruction (e.g., cryotherapy, electrosurgery, ablative laser, or surgical removal), chemical destruction (e.g., salicylic acid or trichloroacetic acid), and anti-proliferative agents (e.g., podophyllin, 5-fluorouracil or bleomycin). Unfortunately, no treatment has yet shown 100% effectiveness as a cure. Furthermore available modalities may cause pain, scarring, and is associated with high recurrence rates.3
Immunotherapeutic agents act by enhancing the host cell-mediated immunity that helps to eliminate the virus rather than simply destroying visible skin lesions4 and have recently received increasing attention for the treatment of warts because of their non-destructive action, high safety profiles, promising results, and low recurrence rates. Contact immunotherapy using contact sensitizers (diphenylcyclopropenone or dinitrochlorobenzene), topical imiquimod, oral cimetidine or intralesional immunotherapy has been attempted as viable immunotherapeutic options for treatment of warts, and their therapeutic effects vary from study to study.
Intralesional immunotherapy has been assessed as an alternative therapeutic approach, particularly for cases of recalcitrant or multiple warts, since it may facilitate the clearance of not only the injected wart but also surrounding non-injected warts. Various immunotherapeutic agents including skin test antigens (mumps, Candida, and Trichophyton); the combined measles, mumps, and rubella vaccine; the tuberculin purified protein derivative; Mycobacterium w vaccine; and bacillus Calmette–Guérin vaccine have been assessed. A recent study used network meta-analysis to examine the comparative efficacy and safety of different modalities in the treatment of warts,5 but the treatment response rate has not been studied systematically. Therefore, we performed a systematic review and meta-analysis of all relevant prospective studies available to evaluate the treatment responses, safety and recurrence rate of each type of intralesional immunotherapy for the management of non-genital warts.
Methods
Protocol and registration
We conducted a single-arm meta-analysis of prospective studies on the treatment response of intralesional immunotherapy for treating non-genital warts. We reported this study following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines6 and this study was registered with PROSPERO, an international database of prospectively registered systematic reviews (https://www.crd.york.ac.uk/PROSPERO/, CRD42020163379).
Databases
We performed a comprehensive search using predefined search terms in MEDLINE, EMBASE, Web of Science, and Cochrane Library databases from the respective dates of database inception to January 4, 2020. The main keywords used were “intralesional,” “immunotherapy,” “purified protein derivative,” “Trichophyton,” “BCG,” “bacillus Calmette-Guerin,” “MMR,” “measles-mumps-rubella,” “candida,” “Mycobacterium,” “vitamin D,” “Corynebacterium,” “INF,” “interferon,” “Propionium,” “Propionibacterium,” “tuberculin,” “vaccine,” “vaccination,” “wart” and “verrucae.” All prospective and experimental studies were included and the reference lists of identified relevant review articles were scanned manually as well. All articles identified using this search process were screened independently by two reviewers (H. J. J. and H. R. P.). In cases of discrepancy between the two main reviewers, a final decision was reached by consensus with two other reviewers (J.M.B. and J.H.L).
Study selection
All relevant clinical studies that reported the treatment response of intralesional immunotherapy by a single injection of the immunotherapeutic agent into the largest lesion in each treatment session were examined. The inclusion criteria were1 prospective study design (randomized or non-randomized controlled trials and open trials);2 participants of all age groups with a diagnosis of a non-genital wart;3 at least one intralesional immunotherapy or control (saline or sterile water injection) group;4 at least 10 participants in each treatment arm, regardless of dropout rate; and,5 outcomes measured based on complete response or no response (0 or <25%). Conversely, the exclusion criteria were1 retrospective or observational study design;2 different outcome measures;3 other interventions or combined; and4 unavailability of the corresponding authors. We excluded studies involving genital warts, as the causative virus and conventional therapeutic modalities are different from those of non-genital warts.
Data extraction, quality assessment and outcome measures
For the meta-analysis, two reviewers extracted the following predefined variables: authors, country, year of publication, study type, the immunotherapeutic agent used, numbers of treated patients, treatment protocols and outcome. Quality assessment of the analytic studies was performed.
The primary outcome was the treatment response rate of intralesional immunotherapy in patients with non-genital warts. Complete response was defined as the complete disappearance of all lesions including both the injected and nearby satellite lesions. The treatment response rate was calculated as the number of participants who achieved complete response divided by the total number of participants who completed the individual study. The no response rate was assessed by dividing the total number of patients who had no or minimal treatment response (<25%) by the total number of participants who had completed the individual study. Because the immune reaction to the intralesional injection can be effective not only for adjacent warts but also for anatomically distant warts, a secondary outcome, defined as distant complete response, was the clearance of distant warts located in an anatomically different body part, away from the injection site. In addition, the recurrence rate was analysed by compiling the studies that reported recurrence. Studies with fewer than 10 patients showing complete response after treatment were excluded.
Meta-regression for age and sex
Meta-regression analyses were conducted to determine whether the estimated treatment response rates varied according to age or sex of patients and were performed by setting the mean age and female to male ratio of participants in each study as moderator variables, respectively.
Safety profiles
We noted all reported adverse events. We recorded common events and their frequencies for each study and searched for serious adverse events.
Statistical analyses
The rates of corresponding treatment responses of the included studies were pooled by generic inverse variance weighting and were combined using a random-effects model.7 Heterogeneity was assessed using the I2 value and subset analyses.8 We used a funnel plot of sample size against log odds to determine publication bias because conventional funnel plots can be asymmetric in the absence of publication bias, especially in studies for extreme proportional metrics.9 Meta-analyses were conducted using R (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria) software with “metagen” and “metafor” packages.
Results
Search results
We initially identified a total of 1,348 records through database searching and six additional records from the reference lists of related articles; 727 duplicates were removed and 494 were deleted after reviewing the titles and abstracts [Figure 1]. A total of 133 full-text articles was assessed in terms of eligibility; of these, 79 were excluded after full-text evaluation. The remaining 54 studies fulfilled the inclusion criteria and were included in the final analysis [Table 1].

- Flow diagram showing how eligible studies were identified in the present review
| Study, yr | Country | Study designa | Population | Enrolled patients, n | Age, yr (range)b | Male/female | Type of wartsc | Immunotherapeutic agentd |
|---|---|---|---|---|---|---|---|---|
| Israel et al., 1969 | US | PD RCT | NA | T: 50C: 50 | NA | NA | NA | T: Smallpox vaccineC: Saline |
| Gibson et al., 1986 | UK | PD OT | Adults | T: 16 | 35 (19–54) | 7/5 | NA | T: IFN-α |
| Vance et al., 1986 | US | PD RCT | NA | T: 80C: 42 | NA | NA | PW | T: IFN-αC: Sterile water |
| Brodell et al., 1995 | US | SA OT | All | T: 22 | 21.9 (7–48) | 9/13 | PW | T: IFN-α |
| Johnson et al., 2001 | US | PD RCT | All | T1: 54 T2: 10 |
31.3 | NA | CW | T1: Mumps antigen T2: Candida antigen |
| Park et al., 2002 | Korea | PD RCT | All | T: 10 | 18.6 (5–37) | 6/4 | PW | T: IFN-α |
| Signore et al., 2002 | US | PD OT | All | T: 100 | 23.7 | 35/52 | CW, PW | T: Candida |
| Clifton et al., 2003 | US | SA OT | Children | T: 47 | 12.9 (4–18) | 25/22 | NA | T: Mumps or Candida antigen |
| Arcaute et al., 2004 | Mexico | SA OT | All | T: 30 | NA (5–67) | 16/14 | NA | T: Candida |
| Johnson et al., 2004 | US | SA OT | All | T: 260 | 21.4 (2–70) | 98/108 | NA | T: Mumps, Candida and Trichopyton antigen |
| Horn et al., 2005 | US | PD RCT | NA | T1: 58 T2: 43 T3: 48 C: 61 |
37 (23–31) 38 (12–29) 40 (21–25) 34 (28–32) |
23/31 12/29 21/25 28/32 |
NA | T1: Mumps or Candida or Trichophyton antigen T2: Antigen plus IFN-α T3: IFN-α C: Saline |
| Aksakal et al., 2008 | Turkey | PD OT | All | T: 45 C: 8 |
25.1 24.6 |
22/23 4/4 |
CW, PW | T: IFN-α C: Saline |
| Kim et al., 2010 | US | SA OT | Adults | T: 18 | 30.18 (18–46) | 6/5 | CW | T: Candida |
| Nofal et al., 2010 | Egypt | PD RCT | Adults | T: 85 C: 50 |
32.4 (14–57) 30.2 (16–52) |
31/39 17/23 |
CW | T: MMR C: Saline |
| Choi et al., 2012 | Korea | SA OT | Children | T: 40 | NA | NA | NA | T: MMR |
| Nasser et al., 2012 | Brazil | PD DB RCT | All | T: 14 C: 14 |
NA | NA | CW, PW | T: Propionium C: Saline |
| Majid et al., 2013 | India | SA OT | Adults | T: 40 | 24.3 (14–36) | 20/14 | CW, PW | T: Candida |
| Meena et al., 2013 | India | SA OT | Adults | T: 40 | 25.2 ± 7.18 (13–48) | 36/4 | CW, FW, PW | T: Mycobacterium |
| Abd-Elazeim et al., 2014 | Egypt | PD RCT | All | T: 20 C: 20 |
22.7 ± 8.4 | 18/22 | CW, PW | T: PPD C: Saline |
| Dogra et al., 2014 | India | PD RCT | NA | T: 33 | NA | NA | CW | T: Mycobacterium |
| Garg et al., 2014 | India | SA OT | All | T: 30 | 22.5 ± 11.1 (6–45) | 19/11 | PW | T: Mycobacterium |
| Zamanian et al., 2014 | Iran | PD DB RCT | All | T: 30 C: 30 |
18.9 ± 12 20.1 ± 10 |
13/11 12/10 |
NA | T: MMR C: Saline |
| Nofal et al., 2015 | Egypt | SA OT | Adults | T: 70 | 38.9 (18–55) | 35/30 | CW | T: MMR |
| Dhakar et al., 2016 | India | PD RCT | Adults | T: 33 | 22.8 | 18/15 | PW | T: Mycobacterium |
| El-Samahy et al., 2016 | Egypt | SA OT | All | T: 52 | 24.6 ± 10.1(5-43) | 12/13 | NA | T: PPD |
| Kerure et al., 2016 | India | SA OT | Adults | T: 110 | 24 (12–52) | NA | CW | T: PPD |
| Parmar et al., 2016 | India | SA OT | Children | T: 44 | NA (4–17) | 17/23 | CW | T: MMR |
| Saini et al., 2016 | India | SA OT | All | T: 100 | 24.8 ± 7.7 (10–45) | 54/32 | CW, FW, PW | T: MMR |
| Saoji et al., 2016 | India | SA OT | All | T: 61 | 28.3 (4–57) | 40/15 | CW, FW, PW | T: PPD |
| Kavya et al., 2017 | India | SA OT | Adults | T: 42 | 20 ± 9.7 (12–66) | 27/15 | CW, PW, filiform wart | T: Vitamin D3 |
| Khozeimeh et al., 2017 | India | PD RCT | Adults | T: 30 | 23.4 ± 6.7 | 19/11 | CW, PW | T: Candida |
| Nofal et al., 2017 | India | SA OT | All | T: 54 | 25.9 ± 13.8 (3–64) | 21/33 | CW | T: Candida |
| Raghukumar et al., 2017 | India | SA OT | All | T: 64 | 23.9 (8–66) | 32/28 | CW, PW, FW, filiform wart | T: Vitamin D3 |
| Agrawal et al., 2018 | India | PD DB RCT | All | T: 50 C: 50 |
25 ± 9.5 (10–45) 27 ± 8.9 (10–44) |
19/11 17/13 |
CW | T: MMR C: Saline |
| Awal et al., 2018 | India | PD RCT | Adults | T: 75 C: 75 |
28.9 ± 9.4 (15–48) 33.6 ± 9.2 (17–50) |
40/32 27/23 |
CW | T: MMR C: Saline |
| Mohtashim et al., 2018 | India | SA OT | Adults | T: 200 | 26.26 ± 8.8 | NA | CW, PW | T: MMR |
| Munnangi et al., 2018 | India | PD OT | Adults | T1: 15 T2: 15 |
21.96 ± 6.79 | 17/13 | CW | T1: MMR T2: BCG |
| Nofal et al., 2018 | Egypt | PD OT | Adults | T: 36 | NA | NA | CW, PW | T1: Candida and acitretin T2: Candida |
| Sabry et al., 2018 | Egypt | SA OT | NA | T: 60 | 21.93 ± 14.24 | 24/34 | CW, PW, FW, filiform wart | T: Candida |
| Abd El-Magid et al., 2019 | Egypt | PD RCT | NA | T1: 39 T2: 39 |
NA (10–60) NA (16–57) |
18/2 16/4 |
PW | T1: Vitamin D3 T2: Zinc |
| Abou-Taleb et al., 2019 | Egypt | PD RCT | Adults | T1: 24 T2: 24 |
31.13 ± 6.86 32.13 ± 13.34 |
18/4 13/10 |
CW, PW | T1: Vitamin D3 T2: PPD |
| Chauhan et al., 2019 | India | SA OT | Adults | T: 110 | 31.31 ± 1.15 (19–62) | 61/49 | CW, PW | T: MMR |
| ElGhareeb et al., 2019 | Egypt | PD OT | Adults | T: 40 | NA | NA | NA | T: MMR |
| El-Taweel et al., 2019 | Egypt | SA OT | Adults | T: 20 | 28.8 (15–50) | 14/6 | CW, PW | T: Vitamin D |
| Hodeib et al., 2019 | Egypt | PD OT | All | T: 20 | 18.9 ± 7.7 (5–40) | 7/13 | FW | T: Candida |
| Jaisinghani et al., 2019 | India | SA OT | Adults | T: 40 | 25.5 (18–46) | 34/0 | CW | T: BCG |
| Kareem et al., 2019 | Egypt | PD OT | Adults | T: 30 C: 20 |
NA (12–50) | NA | CW | T: Vitamin D C: Saline |
| Milante et al., 2019 | Philippines | PD RCT | Adults | T: 29 | 30.66 ± 1.49 | 16/13 | CW, PW | T: PPD |
| Fathy et al., 2019 | Egypt | PD OT | NA | T1: 20 T2: 20 C: 20 |
29.2 26.15 NA |
9/11 15/5 NA |
NA | T1: Candida T2: Vitamin D C: Saline |
| Nasr et al., 2019 | Egypt | SA OT | All | T: 48 | NA (9–45) | 16/32 | NA | T: Candida |
| Rezai et al., 2019 | Iran | PD RCT | NA | T: 30 C: 30 |
27.2 ± 8.73 25.37 ± 9.23 |
12/18 11/19 |
PW | T: MMR C: Saline |
| Naresh et al., 2019 | India | PD RCT | All | T: 60 | 31 (10–60) | 40/20 | CW, PW, filiform wart | T: Vitamin D |
| Verma et al., 2019 | India | SA OT | Adults | T: 36 | 20 (12–60) | 24/12 | CW, PW, filiform wart | T: Vitamin D |
| Nofal et al., 2020 | Egypt | PD OT | Adults | T: 22 | 29.27 ± 8.7 (16–45) | 12/10 | CW, PW | T: HPV vaccine |
aStudy design: SA, single arm; PD, parallel design; OT, open trial; DB, double-blind; RCT, randomized controlled trial, b Reported as mean (range) unless otherwise indicated, cType of warts: CW: common warts, FW: flat warts, PW: plantar warts, dImmunotherapeutic agent: HPV: human papilloma virus, INF: Interferon, MMR: measles, mumps, rubella vaccine, Mw: Mycobacterium w vaccine, PPD: purified protein derivative vaccine, C: Control group, T: treatment group, NA: not available
Characteristics of included studies
A total of 54 studies of 3,446 enrolled patients was deemed finally eligible [Table 2]. Overall, 13 studies with 747 patients in the MMR group10-22; 12 studies with 436 patients in the Candida group23-34; nine studies with 311 patients in the Vitamin D group25,35-42; six studies with 235 patients in the PPD group38,43-47; six studies with 197 patients in the interferon-α group48-53; four studies with 127 patients in the Mw group54-57; two studies with 49 patients in the BCG group13,58; and one study each of other therapeutic agents of smallpox vaccine,59 mumps antigen,34 mumps or Candida antigen,60 mumps or Candida or Trichophyton antigen,52 HPV,61 Propionium,62 and zinc37 were included. As a control group, 13 studies with 470 patients who received saline or sterile water injection were included.10,15,16,20,22,25,40,43,49,52,53,59,62 The mean treatment duration was 8.2 ± 6.0 weeks, and the mean follow-up duration was 4.6 ± 3.5 months (range, 1–25 months).
| Study, yr | Country | Intervention | Enrolled patients, n | Clinical outcomesb, n | Recurrence | Adverse events (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Injected sitea | Session, n* | Interval, wk | Duration, wk† | Follow up, month† | CR | DCR | NR | |||||
| 1. MMR | ||||||||||||
| A Nofal and E Nofal, 2010 | Egypt | MW | 5 | 2 | 8 | 6 | 85 | 57 | 17 | 6 | 0 | Flu-like symptom(8.6%), pain(85.7%) |
| Choi et al., 2012 | Korea | MW | 6 | 2 | 10 | 1 | 40 | 8 | NA | 16 | NA | Flu-like symptom(2.5%), pain(100%) |
| Zamanian et al., 2014 | Iran | EW | 3 | 2 | 4 | 6 | 30 | 18 | NA | NA | NA | Flu-like symptom(30%), pain(100%) |
| Nofal et al., 2015 | Egypt | MW | 5 | 2 | 8 | 6 | 70 | 41 | 38 | NA | 2 | Oedema(1.5%), erythema(4.6%), flu-like symptoms(12.3%), pain(100%), pruritus(6.1%) |
| Parmar et al., 2016 | India | MW | 5 | 3 | 12 | 6 | 44 | 35 | NA | 0 | 0 | Erythema, pain, urticaria |
| Saini et al., 2016 | India | MW | 3 | 3 | 6 | 6 | 100 | 40 | NA | 28 | 3 | Erythema(8.1%), pain(53.5%), post-inflammatory hyperpigmentation(5.8%) |
| Agrawal et al., 2018 | India | MW | 3 | 3 | 6 | 6 | 50 | 18 | 16 | NA | 3 | Erythema(13.3%), pain(60%) |
| Awal and Kaur, 2018 | India | MW | 5 | 2 | 8 | 4 | 75 | 49 | NA | NA | 2 | Oedema/erythema/pruritus(4%), flu-like symptom(6%), pain(90%) |
| Mohtashim et al., 2018 | India | MW | 5 | 2 | 8 | 6 | 200 | 120 | NA | 63 | NA | Pain |
| Munnangi et al., 2018 | India | MW | 5 | 2 | 8 | 3 | 15 | 5 | 3 | NA | NA | Erythema(6%), hyperpigmentation(4%) |
| Chauhan et al., 2019 | India | MW | 5 | 2 | 8 | 2 | 110 | 42 | NA | 7 | 0 | Pain(100%) |
| ElGhareeb et al., 2019 | Egypt | MW | 4 | 2 | 6 | 40 | 29 | 8 | NA | NA | Flu-like symptoms(12.5%) | |
| Rezai et al., 2019 | Iran | MW | 5 | 2 | 8 | 6 | 30 | 14 | NA | NA | 0 | Oedema, erythema, flu-like symptom, pain, pruritus |
| 2. Candida | ||||||||||||
| Johnson et al., 2001 | US | MW | 3 | 3 | 6 | 4 | 10 | 7 | NA | 1 | NA | Flu-like symptom, pain, pruritus |
| Signore, 2002 | US | MW | 3 | 5 | 8 | 25 | 100 | 44 | NA | 8 | NA | Digital oedema(2%), flu-like symptom(5%), headache(1%), herpes zoster(1%), localized wheal(3%), milia(1%), pain(2%), tenderness for 1 week(1%) |
| Arcaute et al., 2004 | Mexico | NA | 2 | 4 | 4 | NA | 30 | 13 | NA | 6 | NA | NA |
| Kim et al., 2010 | US | MW | 10 | 3 | NA | 6 | 18 | 9 | NA | 1 | 1 | Erythema, pain |
| Majid et al., 2013 | India | MW | 3 | 3 | 6 | 6 | 40 | 19 | 3 | NA | 0 | Flu-like symptom(7.5%), pain |
| Khozeimeh et al., 2017 | Iran | MW | 3 | 3 | 6 | NA | 30 | 23 | NA | 6 | NA | Erythema(16.7%), flu-like symptom (3.3%), pain(100%) |
| Nofal et al., 2017 | Egypt | MW | 5 | 2 | 8 | 6 | 54 | 37 | 9 | 5 | 0 | Burning sensation(7.4%), oedema(37%), erythema(9.3%), flu-like symptom(7.4%), pain(100%), pruritus(12.9%) |
| Nofal et al., 2018 | Egypt | MW | 5 | 2 | 8 | 6 | 36 | 12 | NA | NA | NA | Cheilitis, oedema, flu-like symptom, pain, pruritus |
| Sabry et al., 2018 | Egypt | MW | 6 | 2 | 10 | 6 | 60 | 44 | 6 | NA | 4 | Oedema/erythema/pruritus(44.8%), fever(6.9%) |
| Fathy et al., 2019 | Egypt | MW | 3 | 3 | 6 | 6 | 20 | NA | NA | 4 | 2 | Oedema/erythema(25%), pain(100%) |
| Hodeib et al., 2019 | Egypt | MW | 4 | 2 | 6 | 2 | 20 | 12 | NA | 8 | 0 | Oedema(35%), fever(20%), flu-like symptom(25%), hypopigmentation(5%), pain(100%), pain within the day of injection(20%) |
| Nasr et al., 2019 | Egypt | NA | 6 | 2 | 10 | 6 | 48 | 30 | NA | NA | 0 | Burning sensation(10.4%), oedema(20.8%), erythema(41.7%), flu-like symptom(7.4%), pain(100%), pruritus(20.8%) |
| 3. PPD | ||||||||||||
| Abd-Elazeim et al., 2014 | Egypt | MW | 6 | 1 | 5 | 6 | 20 | 9 | NA | 1 | 1 | Oedema (5%), erythema and pain(15%), post-hypopigmentation(10%) |
| El-Samahy et al., 2016 | Egypt | MW | 3 | 3 | 6 | NA | 52 | 9 | NA | 2 | NA | Oedema/erythema/pain(32.7%), pain required the intake of NSAID(3.8%) |
| Kerure et al., 2016 | India | MW | 6 | 2 | 10 | 3 | 110 | 84 | NA | 5 | 0 | Pain |
| Saoji et al., 2016 | India | EW | 4 | 2 | 6 | 6 | 61 | 42 | NA | NA | 1 | Oedema/erythema(21.3%), flu-like symptom(1.6%), eczema(1.6%) |
| Abou-Taleb et al., 2019 | Egypt | MW | 3 | 3 | 6 | 3 | 24 | 13 | 13 | 0 | 0 | Oedema(63.6%), erythema(68.2%), pain(81.8%) |
| Milante et al., 2019 | Philippines | MW | 6 | 2 | 12 | 6 | 66 | 17 | NA | NA | 0 | Constitutional symptoms(9.1%), oedema(10.6%), vesiculation(1.5%) |
| 4. IFN-a | ||||||||||||
| Gibson et al., 1986 | UK | MW | 35 | 1 | 12 | 1.5 | 16 | 11 | NA | NA | NA | Pain, swelling and redness, headache, tiredness, fever, shivering and sweating, aching, stiffness in muscles and joints, sore throat, dizziness, depression, diarrhea, vomiting |
| Vance et al., 1986 | US | MW | 3 | 5 | 3 | 3 | 80 | 11 | NA | 14 | NA | Pain(33.8%) |
| Brodell and Bredle, 1995 | US | MW | 32 | 5 | 8 | 9.5 | 22 | 16 | NA | NA | 3 | Mild discomfort(13.6%), lymphangitis(4.5%) |
| Park et al., 2001 | Korea | MW | 9 | 5 | 3 | 6 | 10 | 5 | NA | NA | 1 | Flu-like symptom(50%), pain(100%) |
| Horn et al., 2005 | US | MW | 5 | 3 | 12 | 0 | 48 | 12 | 3 | 34 | NA | Oedema/erythema(23.9%), flu-like symptom(19.1%) |
| Aksakal et al., 2008 | Turkey | MW | 1 | 0 | 0 | 12 | 45 | 25 | NA | NA | 0 | Flu-like symptom(71.1%) |
| 5. Vitamin D | ||||||||||||
| Kavya et al., 2017 | India | MW | 4 | 2 | 6 | 6 | 42 | 33 | NA | 0 | 1 | Oedema(78.57%), dyspigmentation (2.4%), |
| Raghukumar et al., 2017 | India | EW | 4 | 3 | 9 | 6 | 64 | 54 | NA | NA | 2 | Oedema(3.33%), erythema(5%), pain(100%) |
| Abd El-Magid et al., 2019 | Egypt | EW | 4 | 2 | 8 | 3 | 39 | 2 | NA | 0 | 0 | Hematoma(5%), pain(5%), vasovagal attack(40%) |
| Abou-Taleb et al., 2019 | Egypt | MW | 3 | 3 | 6 | 3 | 24 | 5 | NA | 3 | 0 | Oedema(13%), erythema(17.4%), pain(87%), pruritus(34.8%) |
| El-Taweel et al., 2019 | Egypt | EW | 2 | 4 | 4 | 3 | 20 | 8 | 3 | 3 | 0 | Oedema/erythema(90%), erosion(5%), lymphadenopathy(5%), pain(100%) |
| Fathy et al., 2019 | Egypt | MW | 3 | 3 | 6 | 6 | 20 | NA | NA | 6 | 1 | Pain(100%) |
| Kareem et al., 2019 | Egypt | MW | 2 | 4 | 4 | 3 | 30 | 12 | 2 | 11 | 0 | Pain(23.3%), pruritus(26.7%), both pain and pruritus(10%) |
| Naresh, 2019 | India | EW | 3 | 4 | 9 | 6 | 60 | 48 | NA | NA | 4 | Oedema(60%), Pain(100%) |
| Verma et al., 2019 | India | MW | 2 | 4 | 6 | 6 | 36 | 25 | NA | NA | NA | Oedema(55.5%), dyspigmentation(5.6%) |
| 6. Mycobacterium | ||||||||||||
| Meena et al., 2013 | India | EW | 10 | 1 | 12 | NA | 40 | 23 | NA | 3 | 3 | Oedema(16%), erythema(70%), fever(5%), superficial ulceration(2.5%), tenderness and swelling of submandibular lymph nodes(5%) |
| Dogra et al., 2014 | India | MW | 12 | 1 | 11 | NA | 33 | 20 | NA | NA | NA | NA |
| Garg and Baveja, 2014 | India | MW | 10 | 4 | 36 | 6 | 30 | 28 | NA | 2 | 4 | Oedema/erythema(33.33%), fever(66.67%), headache(10%), myalgia(23.33%), spontaneous ulceration (6.67%), vomiting(6.67%) |
| Dhakar et al., 2016 | India | MW | 12 | 1 | 11 | 4 | 33 | 20 | 12 | 7 | 0 | Cellulitis of lower limb(6.6%), erythematous swelling(73.3%), fever(43.3%), pain(23.3%), regional lymphadenopathy(10%), swelling at the sensitization site(100%) |
| 7. BCG | ||||||||||||
| Munnangi et al., 2018 | India | MW | 5 | 2 | 8 | 3 | 15 | 4 | 1 | NA | NA | Flu-like symptoms(30%), hyperpigmentation(53.3%), Ulceration(60%) |
| Jaisinghani et al., 2019 | India | MW | 3 | 3 | 6 | 3 | 40 | 25 | 9 | 1 | 0 | BCGitis(2.9%), oedema(5.9%), erythema(8.8%), flu-like symptom(100%), hypopigmentation(5.9%), nodule/granuloma(11.8%), pain(100%), pruritus(38.2%), scarring(14.7%), ulceration(5.9%) |
| 8. Others | ||||||||||||
| HPV vaccine | ||||||||||||
| Nofal et al., 2020 | Egypt | MW | 6 | 2 | 10 | 6 | 22 | 18 | 8 | 2 | 0 | Drowsiness or fatigue(9.1%), pain(100%), pruritus(90.9%) |
| Mumps | ||||||||||||
| Johnson et al., 2001 | US | MW | 3 | 3 | 6 | 4 | 45 | 22 | 14 | 4 | 0 | Flu-like symptom, pain, pruritus |
| Mumps or Candida | ||||||||||||
| Clifton et al., 2003 | US | MW | 3 | 3 | 6 | 0 | 47 | 22 | 19 | 9 | NA | Oedema/erythema(10%), pruritus(50%) |
| Mumps or Candida or Trichophyton | ||||||||||||
| Horn et al., 2005 | US | MW | 5 | 3 | 12 | 0 | 58 | 29 | 15 | 25 | NA | Oedema/erythema(26.1%), flu-like symptom(14.9%) |
| Mumps and Candida and Trichophyton | ||||||||||||
| Johnson and Horn, 2004 | US | MW | 10 | 4 | 36 | 0 | 260 | 146 | 112 | 33 | NA | Oedema/erythema/pruritus (20.4%), flu-like symptom(13.6%) |
| Mumps or Candida or Trichophyton and IFN-a | ||||||||||||
| Horn et al., 2005 | US | MW | 5 | 3 | 12 | 0 | 43 | 28 | 20 | 13 | NA | Oedema/erythema(26.1%), flu-like symptom(63.8%), |
| Smallpox vaccine | ||||||||||||
| Israel, 1969 | US | MW | 1 | 0 | 0 | 2 | 50 | 26 | NA | NA | 2 | Erythema/tenderness(40%), lymphangitis(8%), lymphadenitis(8%), malaise and fever(12%) |
| Propionium | ||||||||||||
| Nasser, 2012 | Brazil | MW | 5 | 4 | 20 | 0 | 14 | 8 | NA | 1 | NA | NA |
| Zinc | ||||||||||||
| Abd El-Magid et al., 2019 | Egypt | EW | 4 | 2 | 8 | 3 | 39 | 4 | NA | 0 | 2 | Oedema(15%), hematoma(55%), pain(100%), post-treatment hyperpigmentation(10%), superficial necrosis(15%) |
| 9. Control | ||||||||||||
| Israel, 1969 | US | MW | 1 | 0 | 0 | 2 | 50 | 21 | NA | NA | 0 | Oedema/erythema(2%) |
| Vance et al., 1986 | US | MW | 3 | 5 | 3 | 3 | 42 | 8 | NA | 17 | NA | None |
| Horn et al., 2005 | US | MW | 5 | 3 | 12 | 0 | 61 | 13 | 9 | 47 | NA | Oedema/erythema(23.9%), flu-like symptom(2.1%) |
| Aksakal et al., 2008 | Turkey | MW | 1 | 0 | 0 | 3 | 8 | 0 | NA | NA | 0 | None |
| A Nofal and E Nofal, 2010 | Egypt | MW | 5 | 2 | 8 | 6 | 50 | 11 | 3 | 13 | 3 | None |
| Nasser, 2012 | Brazil | MW | 5 | 4 | 20 | 0 | 14 | 0 | NA | 9 | NA | NA |
| Abd-Elazeim et al., 2014 | Egypt | MW | 6 | 1 | 5 | 6 | 20 | 0 | NA | 18 | 2 | Oedema (5%), erythema and pain(15%), post-hypopigmentation(10%) |
| Zamanian et al., 2014 | Iran | EW | 3 | 2 | 4 | 6 | 30 | 6 | NA | NA | NA | Pain(100%) |
| Agrawal et al, 2018 | India | MW | 3 | 3 | 6 | 6 | 50 | 7 | 0 | NA | 4 | Erythema, pain |
| Awal and Kaur, 2018 | India | MW | 5 | 2 | 8 | 4 | 75 | 5 | NA | NA | 3 | Flu-like symptom(2%), pain(88%) |
| Fathy et al., 2019 | Egypt | MW | 3 | 3 | 6 | 6 | 20 | NA | NA | 20 | NA | Pain(100%) |
| Kareem et al., 2019 | Egypt | MW | 2 | 4 | 4 | 3 | 20 | 1 | 0 | 19 | 0 | Pain(20%) |
| Rezai et al., 2019 | Iran | MW | 5 | 2 | 8 | 6 | 30 | 5 | NA | NA | 0 | Oedema, erythema, flu-like symptom, pain, pruritus |
aInjection site: EW: every single wart, MW: mother wart, bClinical outcomes: CR: complete response, DCR: distant complete response, NR: no response, *maximum, †mean, NA, not available
Treatment response of injected and nearby lesions
The overall complete response rate was 60.6% (95% confidence interval: 54.8–66.5%) among 53 studies with 2,548 patients [Figure 2a]. The complete response ratio of the Mw group was 73.1% (95% confidence interval: 55.6–90.6%) in four studies,54-57,63 the Candida group was 62.6% (95% confidence interval: 53.3–71.9%) in 11 studies,23,24,26-34 MMR group was 63.2% (95% confidence interval: 53.1–73.4%) in 13 studies,10-22 PPD group was 62.7% (95% confidence interval: 42.4–83.0%) in six studies,38,43-47 Vitamin D group was 54.2% (95% confidence interval: 32.7–76.0%) in eight studies,35-42 interferon-α group was 51.9% (95% confidence interval: 27.0–76.9%) in six studies,48-53 and BCG group was 50.9% (95% confidence interval: 5.0–96.8%) in two studies.13,58 There was no significant difference depending on the immunotherapeutic agent used (p-value, 0.88). In the control group receiving saline/sterile water injection, the complete response rate was 17.3% (95% confidence interval: 10.0–24.5%) in 12 studies.10,15,16,20,22,40,43,49,52,53,59,62
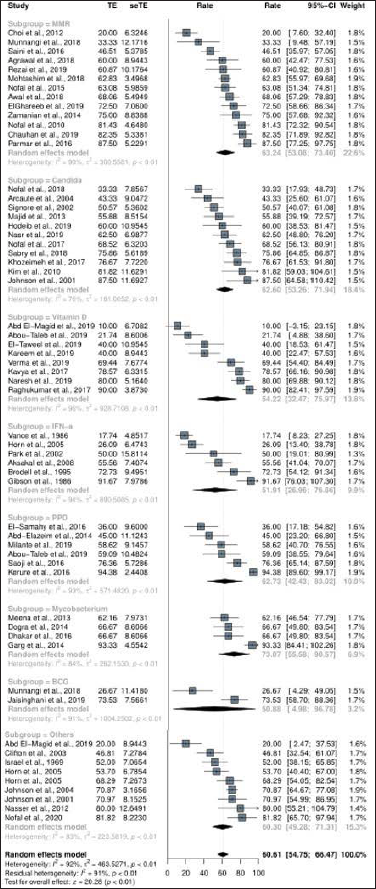
- Treatment response of intralesional immunotherapy. The complete response rate of intralesional immunotherapy on injected and adjacent warts
Overall no response rate of intralesional immunotherapy was 16.6% (95% confidence interval: 12.4–20.8%) in 38 studies including a total of 1,719 patients. The no response rate was highest in the IFN-α group (48.1% [95% confidence interval: -2.2–98.4%] in two studies49,52), followed by in the MMR group (20.9% [95% confidence interval: 7.5–34.2%] in six studies12,14,17,18,21,22) and Candida group (14.6% [95% confidence interval: 8.8–20.3%] in eight studies24,25,28,29,31-34), whereas the no response rate of the control group was 79.7% (95% confidence interval: 66.2–93.2%) in seven studies.22,25,40,43,49,52,62
Treatment response of distant lesions
Among the studies included in this analysis, 15 described the treatment response of distant lesions located away from the mother wart. The overall distant complete response rate of the intralesional immunotherapy was 51.6% (95% confidence interval: 37.9–65.3%) [Figure 2b]. The distant complete response rate of the MMR group was 62.0% (95% confidence interval: 31.1–93.0%) and that of the Candida group was 42.0% (95% confidence interval: 7.3–76.7%). The distant complete response rate of Vitamin D (17.6%, [95% confidence interval: −0.5–35.8%]) and IFN-α (8.8%, [95% confidence interval: −0.7–18.4%]) showed no significant difference from that of the control group with saline injection (14.2% [95% confidence interval: −1.9–30.2%]).
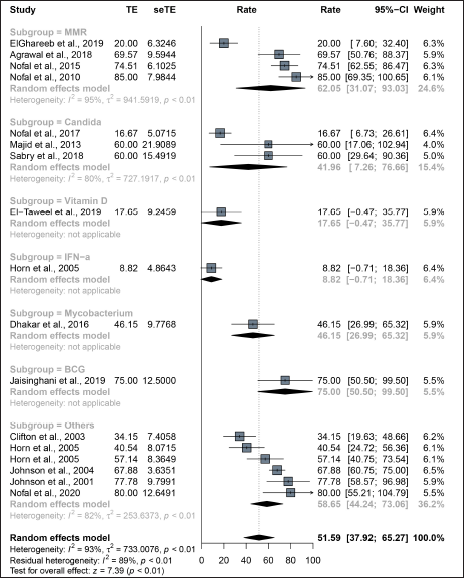
- Treatment response of intralesional immunotherapy. The complete response of intralesional immunotherapy on distant warts located in other body parts
Meta-regression for age and sex
There were no significant linear interactions between mean age and sex (female to male ratio) with changes in treatment response, and the coefficients for the variables were not statistically significant (p-value, 0.61 for age; 0.43 for sex).
Rate of recurrence among patients who have achieved complete response
A total of 47 studies reported recurrence after treatment, and the median follow-up period was six months (range, 0–12 months). The recurrence rates among studies were reported from 0 to 16.7%. The pooled recurrence rate was 2.0% (95% confidence interval: 1.1–2.9%).
Safety of intralesional immunotherapy
Of the 54 clinical studies included in this analysis, 51 reported occurrence of adverse events, and 41 presented the specific frequency of adverse events. The most common adverse event was pain, reported in 35 of 51 studies regardless of the immunotherapeutic agent used, with frequency varying from 2 to 100%. Flu-like symptoms were reported in 22 of the 51 studies, ranging in frequency from 2.5 to 100%. Other adverse events including erythema, oedema, and pruritus have been reported frequently, and lymphadenopathy, vasovagal syncope, dyspigmentation, eczematous reaction and ulceration have been noted as rare adverse events.
Discussion
In this systematic review and meta-analysis, the overall treatment response rate for the complete response of intralesional immunotherapy was 60.6% (95% confidence interval: 54.8–66.5%) [Figure 3]. The complete response rate for each immunotherapeutic agent was observed from 50.9 to 73.1%, but the difference was not statistically significant. The treatment response rate of distant lesions was 51.6% (95% confidence interval: 37.9–65.3%), and all agents except vitamin D and IFN-α showed similar results.
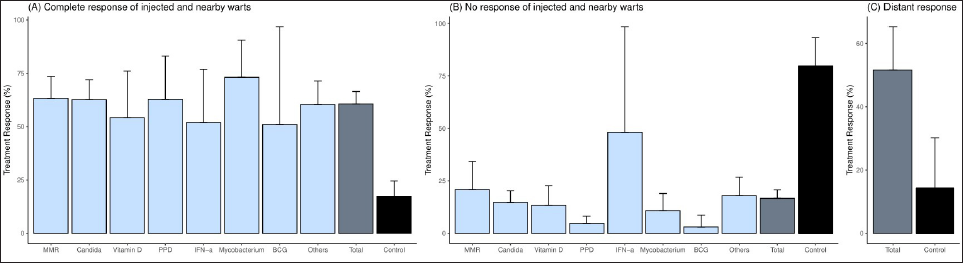
- The pooled treatment response of intralesional immunotherapy
Intralesional immunotherapy is thought to target the CMI response by introducing antigens at the wart site, inducing a T cell-mediated systemic response. All intralesional immunotherapy methods share some common mechanism of action, regardless of the agent used, so it is presumed that they showed similar efficacy in this study. Horn et al. reported that increased proliferation of peripheral blood mononuclear cells to autologous HPV antigens after initiation of intralesional immunotherapy using mumps, Candida, and Trichophyton skin test antigens was more likely to be observed among responders than non-responders.52 Kim et al. in their trial of intralesional injection of Candida antigen reported an immune response to HPV-57 L1 peptide among responders, suggesting that L-1–specific T-cells may be involved in wart regression.31 The strong proinflammatory signals against Mw attract antigen-presenting cells with the production of helper T-cell type 1 cytokines and activation of cytotoxic and natural killer T cells that probably also recognize and process low-profile HPV particles in the infected tissue.64 Vitamin D is thought to be effective in the treatment of warts as a mechanism that regulates cytokine production through its action on Vitamin D receptors at the same time controlling differentiation and proliferation of epidermal cells.65,66
Reports of distant wart resolution suggested a systemic immune response resulting from intralesional immuno therapy. The immunity acquired through the use of an immunotherapeutic method could exert a positive effect with a higher response rate in the treatment of patients with numerous distant warts. An evoked delayed-type hypersensitivity response to both the used antigens and the wart tissue, as well as an instigated cellular immunity through activation of cytotoxic and natural killer cells against HPV have been suggested as aspects of this phenomenon.67 Based on this assumption, the effectiveness of intralesional immunotherapy in eradicating distant warts and the occurrence of better outcomes in patients with previous sensitization to the employed antigens could be justified.22,31,34,60,64
The most troublesome factor in the management of warts is the high recurrence rate of at least 30% after apparently successful treatment, which is possibly driven by the recrudescence of the virus from the surrounding tissue reservoir.68 In the present systematic review, the median follow-up duration among assessed studies was six months, and the pooled recurrence rate was 2.0%, which is remarkably lower than the recurrence rate reported in correlation with conventional treatments. It is assumed that the immune response acquired by intralesional immunotherapy may have played a role in preventing recurrence.
Intralesional immunotherapy is a safe treatment option. The adverse events appeared either in the form of local immunologic or irritant reactions or systemic and constitutional symptoms, such as fever and flu-like symptoms. Pain at the injection site was mentioned in most studies but was rarely prolonged in duration. However, painful indurated nodules, discharges, and scars may occur at the injection site of the Mw vaccine and there was one case report of a severe adverse event of a painful purple finger after injection of Candida albicans antigen for the treatment of a periungual wart,69 so awareness of all possible complications is important.
Intralesional immunotherapy is useful for treatment of non-genital warts, especially in patients with multiple lesions, as it is simple to perform, has a short downtime, rare mild adverse effects, favorable treatment response, and low recurrence rate. Conventional therapies for warts with a destructive mechanism might similarly be effective against conspicuous lesions;70 however, adverse events and high recurrence rates are often major limitations inherent in these approaches.71 For example, the recurrence rate of warts after cryotherapy is as high as 30%.72 Adverse events that follow use of destructive modalities such as infection, ulceration, scarring and hypo- or hyperpigmentation seldom occur when using intralesional antigen immunotherapy.
This study was limited by substantial heterogeneity of the included studies, which may be attributable to variations in treatment regimen, study population race, and disease severity (number, location, and duration of warts) across studies.
Conclusion
We systematically reviewed the response to intralesional immunotherapy in the management of non-genital warts. Intralesional immunotherapy, compared with conventional therapeutic methods, showed favorable treatment outcomes, lower incidence of side effects, and lower recurrence rate. With its efficacy in clearing distant warts, intralesional immunotherapy is a promising treatment approach for patients with multiple or recalcitrant warts.
Abbreviations
BCG: Bacillus Calmette-Guerin
CI: Confidence interval
CMI: Cell-mediated immunity
HPV: Human papillomavirus
MMR: Measles, mumps, and rubella
Mw: Mycobacterium w
PPD: Purified protein derivative
Declaration of patient consent
Patient consent is not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Global burden of human papillomavirus and related diseases. Vaccine. 2012;30:12-23.
- [CrossRef] [PubMed] [Google Scholar]
- Warts are not merely blemishes on the skin: A study on the morbidity associated with having viral cutaneous warts. Australas J Dermatol. 2003;44:169-73.
- [CrossRef] [PubMed] [Google Scholar]
- Therapy of cutaneous human papillomavirus infections. Dermatologic Ther. 2004;17:441-8.
- [CrossRef] [PubMed] [Google Scholar]
- Evolving role of immunotherapy in the treatment of refractory warts. Indian Dermatol Online J. 2016;7:364-70.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional immunotherapy for the treatment of warts: A network meta-analysis. J Am Acad Dermatol. 2019;80:922-30.
- [CrossRef] [PubMed] [Google Scholar]
- Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-88.
- [CrossRef] [PubMed] [Google Scholar]
- Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-60.
- [CrossRef] [PubMed] [Google Scholar]
- In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67:897-903.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional injection of the measles-mumps-rubella vaccine into resistant palmoplantar warts: A randomized controlled trial. Iran J Med Sci. 2019;44:10-17.
- [PubMed] [PubMed Central] [Google Scholar]
- Comparative study of autoimplantation therapy and intralesional injection of MMR vaccine in warts treatment. Dermatol Ther. 2019;32:e13135.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy and safety of intralesional immunotherapy with measles, mumps, rubella virus vaccine for the treatment of common warts in adults. Indian Dermatol Online J. 2019;10:19-26.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative study between intralesional MMR and intralesional BCG in treatment of verruca vulgaris. IOSR J Dent Med Sci. 2018;17:44-50.
- [CrossRef] [Google Scholar]
- Efficacy of intralesional MMR vaccine in treatment of single or multiple refractory cutaneous warts. Przeglad Dermatologiczny. 2018;105:498-508.
- [CrossRef] [Google Scholar]
- Therapeutic outcome of intralesional immunotherapy in cutaneous warts using the mumps, measles, and rubella vaccine: A randomized, placebo-controlled trial. J Clin Aesthet Dermatol. 2018;11:15-20.
- [PubMed] [PubMed Central] [Google Scholar]
- A Randomized double blind controlled study comparing the efficacy of intralesional MMR vaccine with normal saline in the treatment of cutaneous warts. Indian Dermatol Online J. 2018;9:389-93.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional mumps, measles and rubella vaccine in the treatment of cutaneous warts. Indian J Dermatol Venereol Leprol. 2016;82:343-5.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional measles, mumps and rubella vaccine for the treatment of multiple and recalcitrant nongenital cutaneous warts in children: A prospective, uncontrolled open-label study. BJD. 2016;175:160.
- [Google Scholar]
- Treatment of recalcitrant warts with intralesional measles, mumps, and rubella vaccine: A promising approach. Int J Dermatol. 2015;54:667-71.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of intralesional injection of mumps-measles-rubella vaccine in patients with wart. Adv Biomed Res. 2014;3:107.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional immunotherapy of warts with mumps, measles and rubella vaccine. J Dermatol. 2012;39:63-4.
- [Google Scholar]
- Intralesional immunotherapy of common warts: Successful treatment with mumps, measles and rubella vaccine. J Eur Acad Dermatol Venereol. 2010;24:1166-70.
- [CrossRef] [PubMed] [Google Scholar]
- Role of mannose binding lectin in response to candida antigen immunotherapy of warts. J Dermatolog Treat. 2021;32:376-80.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study of intralesional injection of candida albicans antigen, bleomycin and 5-fluorouracil for treatment of plane warts. J Dermatolog Treat. 2021;32:663-8.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional vitamin D3 versus candida antigen immunotherapy in the treatment of multiple recalcitrant plantar warts: A comparative case–control study. Dermatol Ther. 2019;32:e12997.
- [CrossRef] [PubMed] [Google Scholar]
- Peripheral blood toll-like receptor 4 correlates response to candida immunotherapy of warts. Dermatol Ther. 2018;31:e12691.
- [CrossRef] [PubMed] [Google Scholar]
- Combined acitretin and Candida antigen versus either agent alone in the treatment of recalcitrant warts. J Am Acad Dermatol. 2018;79:377-8.
- [CrossRef] [PubMed] [Google Scholar]
- Significance of interferon gamma in the prediction of successful therapy of common warts by intralesional injection of Candida antigen. Int J Dermatol. 2017;56:1003-9.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional immunotherapy compared to cryotherapy in the treatment of warts. Int J Dermatol. 2017;56:474-8.
- [CrossRef] [PubMed] [Google Scholar]
- Immunotherapy with intralesional Candida albicans antigen in resistant or recurrent warts: A study. Indian J Dermatol. 2013;58:360-5.
- [CrossRef] [PubMed] [Google Scholar]
- Phase 1 clinical trial of intralesional injection of candida antigen for the treatment of warts. Arch Dermatol. 2010;146:1431-3.
- [CrossRef] [PubMed] [Google Scholar]
- Experience with intralesional candidin in the treatment of viral warts. Dermatologia Revista Mexicana. 2004;48:307-10.
- [Google Scholar]
- Candida albicans intralesional injection immunotherapy of warts. Cutis. 2002;70:185-92.
- [PubMed] [Google Scholar]
- Intralesional injection of mumps or candida skin test antigens: A novel immunotherapy for warts. Arch Dermatol. 2001;137:451-5.
- [PubMed] [Google Scholar]
- Safety and efficacy of intralesional vitamin D3 in cutaneous warts: An open uncontrolled trial. J Cutan Aesthet Surg. 2017;10:90-4.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional Vitamin D3 injection in the treatment of recalcitrant warts: A novel proposition. J Cutan Med Surg. 2017;21:320-4.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional injection of vitamin D3 versus zinc sulfate 2% in treatment of plantar warts: A comparative study. J Dermatolog Treat. 2021;32:355-60.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional vitamin D3 versus intralesional purified protein derivative in treatment of multiple warts: A comparative clinical and immunological study. Dermatol Ther. 2019;32:e13034.
- [CrossRef] [PubMed] [Google Scholar]
- Cigarette smoking reduces the efficacy of intralesional vitamin D in the treatment of warts. Dermatol Ther. 2019;32:e12816.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of intralesional vitamin D3 injection in the treatment of common warts: Single-blinded placebo-controlled study. Dermatol Ther. 2019;32:e12882.
- [CrossRef] [PubMed] [Google Scholar]
- A study of effectiveness of intralesional vitamin D3 in treatment of multiple cutaneous warts. IOSR J Dent Med Sci. 2019;18:84-7.
- [CrossRef] [Google Scholar]
- Intralesional vitamine-D3 in multiple skin warts. IOSR J Dent Med Sci. 2019;18:74-8.
- [CrossRef] [Google Scholar]
- Evaluation of IL-12 serum level in patients with recalcitrant multiple common warts, treated by intralesional tuberculin antigen. J Dermatolog Treat. 2014;25:264-7.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of serum interleukin-12 and interferon-y in patients with multiple warts treated with intralesional tuberculin injection. J Egypt Women’s Dermatol Soc. 2016;13:23-8.
- [CrossRef] [Google Scholar]
- Intralesional immunotherapy with tuberculin purified protein derivative for verruca: A study from a teaching hospital in South India. Indian J Dermatol Venereol Leprol. 2016;82:420-2.
- [CrossRef] [PubMed] [Google Scholar]
- Immunotherapy using purifified protein derivative in the treatment of warts: An open uncontrolled trial. Indian J Dermatol Venereol Leprol. 2016;82:42-6.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of single versus multiple intralesional immunotherapy with purified protein derivative (PPD) in the treatment of multiple verruca vulgaris. Int J Dermatol. 2019;58:1477-82.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of common and plantar viral warts with human lymphoblastoid interferon-α - Pilot studies with intralesional, intramuscular and dermojet injections. Br J Dermatol. 1986;115:76-9.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional recombinant alpha-2 interferon for the treatment of patients with condyloma acuminatum or verruca plantaris. Arch Dermatol. 1986;122:272-7.
- [PubMed] [Google Scholar]
- The treatment of palmar and plantar warts using natural alpha interferon and a needleless injector. Dermatol Surg. 1995;21:213-8.
- [CrossRef] [PubMed] [Google Scholar]
- Combination therapy with intralesional interferon a-2b and pulsed dye laser for the treatment of periungual warts. Ann Dermatol. 2002;14:82-7.
- [CrossRef] [Google Scholar]
- Intralesional immunotherapy of warts with mumps, Candida, and Trichophyton skin test antigens: A single-blinded, randomized, and controlled trial. Arch Dermatol. 2005;141:589-94.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of verruca plantaris with a single sublesional injection of interferon-alpha2a. Clin Exp Dermatol. 2009;34:16-9.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional immunotherapy with mycobacterium w vaccine in patients with multiple cutaneous warts: Uncontrolled open study. JAMA Dermatol. 2013;149:237-9.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of intralesional mycobacterium w vaccine and cryotherapy in the treatment of refractory extragenital warts: A randomized open-label comparative study. Br J Dermatol. 2014;171:55-6.
- [Google Scholar]
- Intralesional immunotherapy for difficult to treat warts with Mycobacterium w vaccine. J Cutan Aesthet Surg. 2014;7:203-8.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional Mycobacterium w Vaccine Versus cryotherapy in treatment of refractory extragenital warts: A randomized, open-label, comparative study. J Cutan Med Surg. 2016;20:123-9.
- [CrossRef] [PubMed] [Google Scholar]
- Bacillus Calmette-Guerin immunotherapy for recurrent multiple warts: An open-label uncontrolled study. Indian J Dermatol. 2019;64
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of warts by vaccination. Arch Dermatol. 1969;100:222-3.
- [CrossRef] [PubMed] [Google Scholar]
- Immunotherapy for recalcitrant warts in children using intralesional mumps or Candida antigens. Pediatr Dermatol. 2003;20:268-71.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional versus intramuscular bivalent human papillomavirus vaccine in the treatment of recalcitrant common warts. J Am Acad Dermatol. 2020;82:94-100.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of common warts with the immune stimulant Propionium bacterium parvum. An Bras Dermatol. 2012;87:585-9.
- [CrossRef] [PubMed] [Google Scholar]
- A double-blind, randomized controlled trial to compare the effectiveness and safety of purified protein derivative of tuberculin antigen with mycobacterium w vaccine in the treatment of multiple viral warts. Indian J Dermatol Venereol Leprol. 2019;85:355-66.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: An open label pilot study. J Eur Acad Dermatol Venereol. 2008;22:1089-93.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D and systemic cancer: Is this relevant to malignant melanoma? Br J Dermatol. 2002;147:197-213.
- [CrossRef] [PubMed] [Google Scholar]
- Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770-3.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional immunotherapy with tuberculin purified protein derivative (PPD) in recalcitrant wart: A randomized, placebo-controlled, double-blind clinical trial including an extra group of candidates for cryotherapy. J Dermatolog Treat. 2016;27:173-8.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional immunotherapy for the management of warts. Indian J Dermatol Venereol Leprol. 2011;77:261-3.
- [CrossRef] [PubMed] [Google Scholar]
- The painful purple digit: An alarming complication of Candida albicans antigen treatment of recalcitrant warts. Dermatitis. 2005;16:38-40.
- [PubMed] [Google Scholar]
- Cryotherapy of common viral warts at intervals of 1, 2 and 3 weeks. Br J Dermatol. 1995;132:433-6.
- [CrossRef] [PubMed] [Google Scholar]
- Immunotherapy and warts: A point of view. Clin Dermatol. 2008;26:223-5.
- [CrossRef] [PubMed] [Google Scholar]
- An armamentarium of wart treatments. Clin Med Res. 2006;4:273-93.
- [CrossRef] [PubMed] [Google Scholar]






