Translate this page into:
An unusual case of nasal chromoblastomycosis progressing to squamous cell carcinoma in a non-endemic region
Corresponding author: Dr. Refka Frioui, Department of Dermatology, Military Hospital of Instruction of Tunis, Bardo, Tunis, Tunisia. rafkouna1993@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Frioui R, Jaber K, Mtibaa L, Jamli B, Gargouri F, Rabhi F, et al. An unusual case of nasal chromoblastomycosis progressing to squamous cell carcinoma in a non-endemic region. Indian J Dermatol Venereol Leprol 2023;89:102-5.
Sir,
Chromoblastomycosis is a slowly progressive granulomatous mycosis of the skin and subcutaneous tissue, caused by inoculation of dematiaceous fungi mainly affecting the lower limbs. The infection is common in tropical and subtropical regions, but there have been several cases reported in temperate regions.1 Early diagnosis and treatment is necessary as longstanding cases can rarely undergo malignant transformation into squamous cell carcinoma.1 Herein, we report an unusual case of chromoblastomycosis in Tunisia, anon-endemic area,occurring at a distant site from the original lesion and progressing into squamous cell carcinoma.
A 60-year-old man from North Tunisia, a masseur in a Turkish bath presented to our department in 2006 with an infiltrated plaque on the right thigh. He had a history of trauma at the same site two years back at work. There was no history of travel to tropical areas. The histopathological examination revealed the presence of fungal elements. Mycological examination confirmed the diagnosis of chromoblastomycosis but the species was not identified. He was treated with terbinafine (500 mg/day) for three months. There was a significant decrease in the size of the lesion, but he was lost to follow-up. Eight years later, he presented with a reddish fleshy mass in the left nasal cavity associated with mucopurulent discharge. Over the past three months, the mass rapidly increased in size and extended to involve the nasal dorsum. On examination there was a 4 × 3 cm ulcero proliferative nasal tumour with irregular edge surmounted by telangiectasia on the nasal dorsum and left nasal cavity [Figure 1a]. Dermoscopy revealed a vascular pattern with polymorphous vessels [Figure 1b]. Cervical lymph nodes were not palpable. On the right thigh, there was a 12 × 13 cm erythematous to verrucous plaque interspersed with areas of atrophy and depigmentation [Figure 2]. Clinically, the differential diagnoses of tumour form of chromoblastomycosis and squamous cell carcinoma were considered. Biopsies were performed at different sites of the nasal and the thigh lesion. Histological examination of the biopsy specimens taken from the margin of the nasal lesion revealed the presence of fungal elements within a granulomatous reaction with mixed inflammatory infiltrate [Figures 3a and 3b]. The same histopathological findings were noted in the thigh lesion. Other endonasal and nasal dorsum biopsy tissues showed a moderately differentiated squamous cell carcinoma [Figure 3c]. Direct microscopic examination of the nasal specimens showed fumagoid cells [Figure 4a]. Fungal cultures on Sabouraud dextrose agar showed growth of pigmented velvety dark colonies [Figures 4b-4d]. Microscopic examination of the colonies revealed cylindrical septate hyphae, conidiophores swollen at their termination carrying ovoid conidia suggestive of Fonsecaea pedrosoi species [Figure 4e]. The same findings were also seen with the tissues taken from the thigh lesion. Molecular identification of the fungal colonies was performed by internal transcribed spacer (ITS) region, Internal Transcribed Spacer 1 to Internal Transcribed Spacer 4 (ITS1 to ITS4) primers. A 700 bp fragment was amplified from fungal colonies obtained with culture of tissues taken from the nasal lesion and the thigh lesion [Figure 5]. The amplicon from both samples was purified separately and the nucleotide sequence was determined in both the directions. The consensus sequences homology search was performed in the GenBank database.The sequence of the two strains displayed 100% of homology with Fonsecaea monophora. CT scan of the neck, chest and abdomen were normal. Head CT scan showed a left nasal cavity mass.

- Ulceroproliferative nasal tumour with irregular edge surmounted by telangiectasia on the left nasal cavity and nasal dorsum

- Polarised dermosocopy shows vascular pattern with polymorphous vessels (Illuco IDS 1100, ×10 polarised)

- A 12 × 13 cm erythematous to verrucous plaque interspersed with atrophy and depigmentation on the right thigh

- (a) Brownish, round bodies with a thick wall called as sclerotic cells (red arrow) (PAS ×400). (b) Granulomatous dermatitis: tuberculoid granuloma (red circle) (H and E ×200). (c) Moderately-differentiated squamous cell carcinoma: pleomorphic squamous epithelial cells arise from the epidermis and extend into the dermis (H and E ×40).

- (a) Direct examination demonstrated fumagoid cells “Fonscecaea pedrosoi species” scale bar = 10 um; Direct examination demonstrated the presence of darkly pigmented sclerotic bodies with crosswalls (inset) (4b) Colony aspects of culture of the skin part taken from the thigh: pigmented velvety dark colonies (a 15-day culture on Sabouraud dextrose broth) (c) Colony aspects of culture of the biopsy taken from the nasal tumor: pigmented velvety dark colonies (a 10-day culture on Sabouraud dextrose broth) (d) Colony aspects of culture of the biopsy taken from the nasal tumor: pigmented velvety dark colonies (a 15-day culture on Sabouraud dextrose broth) (e) Dematiaceous cylindrical sepate nyphae. Conidia (white arrows) arising from conidiophores (black arrows), arranged in short chains, characteristic of Fonsecaea pedrosoi. Scale bar: 10 um.
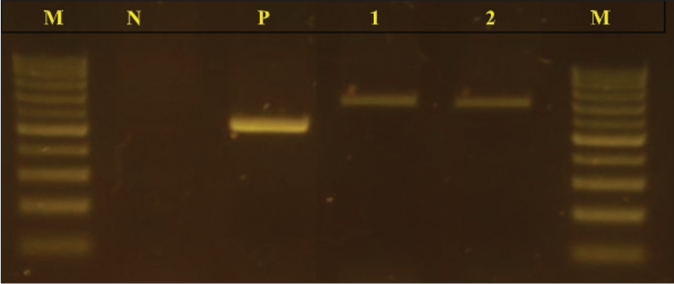
- Agarose gel electrophoresis profile of PCR-amplified products using the primers Internal Transcribed Spacers: Internal Transcribed Spacer 1 to Internal Transcribed Spacer 4. From left: lane M: molecular size marker100 bp DNA ladder; lane N: negative control; lane P: positive control Candida albicans strain; lane 1: fungal isolate of the lesion of the right thigh; lane 2: fungal isolate of the nasal tumour
Based on the above findings, the diagnosis of nasal chromoblastomycosis with squamous cell carcinoma was made. A combination therapy of 500 mg of terbinafine and 200 mg of itraconazole daily was started. He was referred to the oncosurgery department and advised to undergo excision, after which the patient was lost to follow-up.
Chromoblastomycosis is most prevalent in tropical and subtropical climate areas of America, Asia and Africa.2 However, sporadic cases are increasingly described in temperate zones, such as West Europe and North Africa.
There have been five cases of chromoblastomycosis reported till date from Maghreb countries, particularly in Tunisia.3 Chromoblastomycosis is caused by pigmented fungi with low pathogenicity that are thermosensitive at 40–42°C.They live as saprophytes in the soil, plant thorns, debris and transported wood. They have also been isolated in saunas where the conditions of high humidity and heat create a tropical microclimate that might explain the increasing incidence of cases even in temperate areas.4 This could probably be the cause in our patient who worked as a masseur in Turkish bath.
Typical lesions tend to be found usually in exposed areas of the body especially the feet and leg. According to Minotto et al., 27% of the cases involve other areas, that includes medial canthus of the eye, the ear, neck, shoulder, wrist, chestand buttock.5 The nasal cavity is rarely affected by chromoblastomycosis and there have been only a few cases described.6 In our case the initial lesion occurred on the inner part of the thigh which is an unusual location which could be explained due to his profession. Chromoblastomycosis is a localised infection with spread to body sites in the vicinity of the original lesion, without metastasis to distant sites. Thespread to the second affected site in our case might have been caused by autoinoculation due to itching.
Chromoblastomycosis has diverse clinical presentations: nodular, verrucous, tumoral, plaque-like and psoriasiform. Cicatricial atrophy with central sparing may also be seen. Due to the various clinical polymorphism chromoblastomycosis may be confused with leishmaniasis, verrucous form of tuberculosis and tertiary syphilis. The diagnosis of chromoblastomycosis is based on histopathological and mycological tests. Examination of the skin biopsies or scrapings under the microscopy, reveal the pathognomonic ‘muriform cells’. These rounded cigar-coloured and cross-chambered structures are distinctive and are alsoknown as medlar bodies, sclerotic bodies or fumagoid cells. Histologically, a dermal granulomatous infiltrate with a predominance of epithelioid cells surrounding fumagoid bodies is seen. Presumptive species identification may be achieved by mycological examination, but molecular techniques are suggested for definitive identification.
The lesions are usually recalcitrant. If not diagnosed and treated early, the disease has a chronic course with lethal complications.
The major risk is malignant transformation into squamous cell carcinoma.1 The risk of malignant transformation is about 1% as described in a study carried out in Madagascar where neoplasm was reported in 14 out of 1400 cases over 50 years of study.7 The first reported case of malignant transformation was described by Caplan in 1968 in a patient from Nicaragua, presenting with verrucous plaque on both legs, left thig hand right hand which evolved over approximately11years.8 Subsequently, about 23 cases of tumours derived from chromoblastomycosis have been documented worldwide. Sex ratio of male versus female was 21:2. The mean time of malignant transformation was 19.7 years9 The main affected site was lower limbs.9 In majority of the cases squamous cell carcinoma resolved after surgical excision.9
Any atypical changes like development of ulceration, a rapid growth or a poor response to treatment should raise the suspicion of malignancy.Our patient had an asymptomatically growing endonasal chromoblastomycosis that slowly progressed to squamous cell carcinoma.
Acknowledgements
The authors would like to acknowledge the patient.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Chromoblastomycosis associated with a lethal squamous cell carcinoma. An Bras Dermatol. 2010;85:267-70.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular epidemiology of agents of human chromoblastomycosis in brazil with the description of two novel species. PLoS Negl Trop Dis. 2016;10:5102.
- [CrossRef] [PubMed] [Google Scholar]
- Chromoblastomycosis: Solitary lesion of the breast. Presse Méd. 2015;44:842-3.
- [CrossRef] [PubMed] [Google Scholar]
- Chromomycosis in Finland. The possible share of the Finnish sauna in its distribution. Hautarzt. 1966;17:507-9.
- [Google Scholar]
- Chromoblastomycosis: A review of 100 cases in the state of Rio Grande do Sul, Brazil. J Am Acad Dermatol. 2001;44:585-92.
- [CrossRef] [PubMed] [Google Scholar]
- Isolated nasal chromoblastomycosis. Indian J Pathol Microbiol. 2014;57:519-21.
- [CrossRef] [PubMed] [Google Scholar]
- Squamous cell carcinoma arising from chromomycosis. Report of two cases. Ann Pathol. 1999;19:516-20.
- [Google Scholar]
- Epidermoid carcinoma arising in extensive chromoblastomycosis. Arch Dermatol. 1968;97:38-41.
- [CrossRef] [PubMed] [Google Scholar]
- Chromoblastomycosis by Cladophialophora carrionii associated with squamous cell carcinoma and review of published reports. Mycopathologia. 2015;179:153-7.
- [CrossRef] [PubMed] [Google Scholar]





