Translate this page into:
Facial Angiofibroma Severity Index: A beneficial tool to evaluate the treatment response in the skin of colour
Corresponding author: Dr. Sowmya S Aithal, 4753/A, Sindhoora, Opposite Jyothi IVF centre, Dattagalli 3rd stage, Mysore-570033, Karnataka, India. amritahongalleo@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Hongal AA, Aithal SS, Revathi TN. Facial Angiofibroma Severity Index: A beneficial tool to evaluate the treatment response in the skin of colour. Indian J Dermatol Venereol Leprol 2023;89:130-2.
Sir,
Facial Angiofibroma Severity Index is a simple and reliable tool to evaluate the clinical severity and response to the treatment of angiofibromas associated with tuberous sclerosis. It was developed by R. Salido-Vallejo et al. in 2014 as a standardised tool for the measurement of severity of facial angiofibromas.1 For the treatment of angiofibromas, various modalities such as laser, surgery, cryotherapy or dermabrasion have been tried, but they can be painful, cause scarring and do not prevent the recurrence of lesions. Topical sirolimus treatment of tuberous sclerosis complex (TSC)-related facial angiofibromas is suggested as one of the efficacious modalities.2
We report five cases of facial angiofibromas that presented to us in our hospital. After obtaining valid informed consent and regular blood investigations, treatment was started with 0.1% sirolimus cream, which was obtained by powdering 1 mg tablet and combining with a necessary cream base to obtain a topical formulation. It was applied twice daily for three months and once daily as maintenance. The efficacy was evaluated using the Facial Angiofibroma Severity Index. The score was obtained by summing the scores given to each of the three features; erythema scored 0–3, lesion size 1–3 and extent scored 2–3. Two dermatologists assessed photographs independently and the score was obtained as average [Table 1]. Patients were evaluated monthly for three months [Figures 1–4]. The information panel used for scoring erythema, size and extent by Salido Vallejo et al. was used as a guide to scoring Facial Angiofibroma Severity Index in our patients [Figure 5].
| Patient number | Age | Sex | Baseline FASI = Erythema + size + extent | 3rd month FASI = Erythema + size + extent |
|---|---|---|---|---|
| 1 | 32 | Male | 9 = 3 + 3 + 3 | 3 = 0 + 1 + 2 |
| 2 | 14 | Male | 5 = 1 + 2 + 2 | 3 = 0 + 1 + 2 |
| 3 | 26 | Female | 7 = 2 + 2 + 3 | 5 = 1 + 2 + 2 |
| 4 | 23 | Male | 5 = 0 + 3 + 2 | 4 = 0 + 2 + 2 |
| 5 | 28 | Female | 4 = 1 + 1 + 2 | 3 = 0 + 1 + 2 |
Erythema: Skin colour: 0, light red: 1, red: 2, and dark red/purple: 3, Size: Small (<5 mm): 1, large (>5 mm): 2 and Confluent: 3, Extension: <50% cheek area: 2 and >50% cheek area: 3, FASI Score ≤5: mild, 6–7: moderate and ≥8: severe, FASI: Facial Angiofibroma Severity Index

- First patient with baseline Facial Angiofibroma Severity Index-9
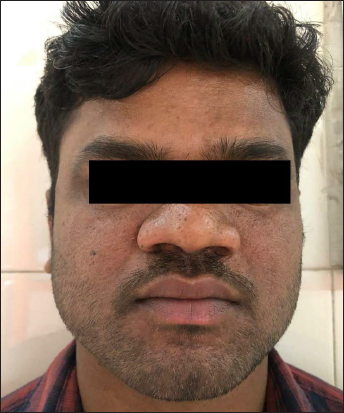
- First patient evaluated at three months, improving to Facial Angiofibroma Severity Index-3 after treatment
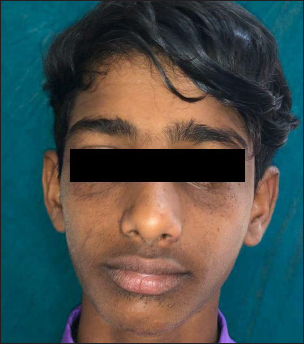
- Second patient with baseline Facial Angiofibroma Severity Index-5
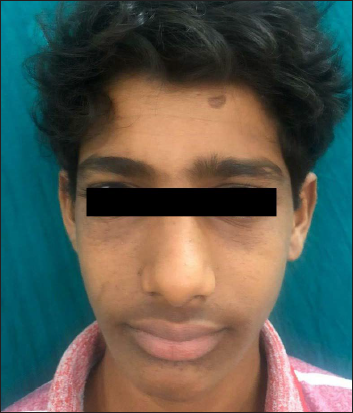
- Second patient improving to Facial Angiofibroma Severity Index-3 after treatment
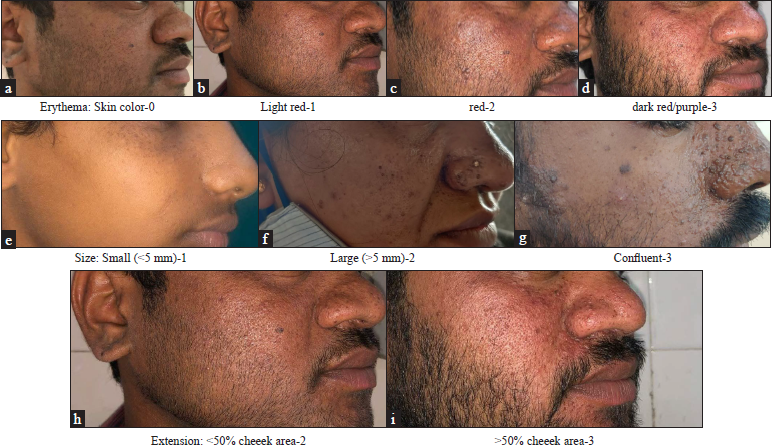
- Illustrative sample images scoring erythema, size and extent
Scoring indices are required to assess the clinical severity and treatment response in different diseases. They help in standardising the evaluation and act as a helpful tool. Facial Angiofibroma Severity Index was developed as a useful and reproducible objective clinical tool to assess the severity of facial angiofibromas and treatment response with good inter-rater reliability.
Facial Angiofibroma Severity Index is obtained by summing the partial scores assigned to three features: erythema, size and extent of lesions. Erythema and lesion size are scored from 0 to 3, while the extent is scored from 2–3 since it carries greater specific weight in estimating the degree of severity. The cheeks are taken as a reference area for evaluating extent. Mild, moderate and severe facial angiofibroma correspond with activity score ranges on the Facial Angiofibroma Severity Index of ≤5, 6–7 and ≥8, respectively.1
Facial angiofibromas are reddish-pink, dome-shaped papules, 1–10 mm in size, distributed over the face, and are of primary cosmetic concern among individuals with Tuberous sclerosis. Angiofibromas show increased expression of angiogenic factors like vascular endothelial growth factor and mammalian target of rapamycin (mTOR) over-activation that promote angiogenesis.
Sirolimus (Rapamycin) is a lipophilic macrocyclic lactone, first isolated from Streptomyces hygroscopicus, which binds to the 12-kDa FK506-binding protein (FKBP12) and inhibits the procarcinogenic action of the mTOR pathway. Inhibition of the mTOR pathway decreases the output of vascular endothelial growth factor by inhibiting hypoxia-inducible factor expression and by directly repressing vascular endothelial growth factor-stimulated endothelial cell proliferation.3 Effective clinical improvement has been reported in various studies using the Facial Angiofibroma Severity Index score after treatment with topical sirolimus.4,5
Koenig et al. compared 1% sirolimus, 0.1% sirolimus and vehicle alone. They found that topical sirolimus is an effective and safe treatment modality for tuberous sclerosis-related facial angiofibromas, and in the trial, the preferred dose was 1% once daily.1
In vitro percutaneous absorption of topical sirolimus is significantly greater with the gel compared with the ointment.6 Common side effects include irritation and a burning sensation.
Only a few Indian studies have used the Facial Angiofibroma Severity Index as an objective tool for evaluation.7,8 Erythema, one of the major components of the Index, is difficult to appreciate in the skin of colour since it is masked by melanin. Other limitations of the Index include the mild degree of inter-observer variability and the fact that it cannot be used to assess unilateral angiofibromas since this system of measurement assesses bilateral and symmetrical angiofibromas.1
The Facial Angiofibroma Severity Index is simple, valid, reproducible and easy to apply in assessing angiofibroma. Despite the difficulty in appreciating erythema in the skin of colour, it serves as a helpful index in evaluating response to treatment. One such efficacious treatment modality is topical sirolimus.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Facial Angiofibroma Severity Index (FASI): Reliability assessment of a new tool developed to measure severity and responsiveness to therapy in tuberous sclerosis-associated facial angiofibroma. Clin Exp Dermatol. 2014;39:888-93.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of topical rapamycin in patients with facial angiofibromas secondary to tuberous sclerosis complex: The TREATMENT randomized clinical trial. JAMA Dermatol. 2018;154:773.
- [CrossRef] [PubMed] [Google Scholar]
- Topical rapamycin (sirolimus) for facial angiofibromas. Indian Dermatol Online J. 2013;4:54.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberous sclerosis complex in 29 children: Clinical and genetic analysis and facial angiofibroma responses to topical sirolimus. Pediatr Dermatol. 2017;34:572-7.
- [CrossRef] [PubMed] [Google Scholar]
- Sirolimus ointment for facial angiofibromas in individuals with tuberous sclerosis complex. Int Sch Res Notices. 2017;2017:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- First left–right comparative study of topical rapamycin vs. vehicle for facial angiofibromas in patients with tuberous sclerosis complex. Br J Dermatol. 2013;169:1314-8.
- [CrossRef] [PubMed] [Google Scholar]
- Use of topical rapamycin in facial angiofibromas in Indian skin type. Indian Journal of Dermatology. 2016;61:119.
- [CrossRef] [PubMed] [Google Scholar]
- Topical sirolimus in facial angiofibroma. Indian Journal of Drugs in Dermatology. 2018;4:76.
- [CrossRef] [Google Scholar]





