Translate this page into:
Dupilumab provides rapid improvement for morphologic variants of paediatric atopic dermatitis: A case series
Corresponding author: Jung Im Na, Associate Clinical Professor, Department of Dermatology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam, Republic of Korea. jina1@snu.ac.kr
-
Received: ,
Accepted: ,
How to cite this article: Kim JW, Kim M, Ahn GS, Na JI. Dupilumab provides rapid improvement for morphologic variants of paediatric atopic dermatitis: A case series. Indian J Dermatol Venereol Leprol 2022;88:834-9.
Sir,
Recent studies have demonstrated that Th2 immune mediators, interleukin-4 and interleukin-13, may play a key role in the development of atopic dermatitis. This has led to the advent of dupilumab for the targeted treatment of refractory atopic dermatitis.1 Morphological variants of atopic dermatitis including nummular eczema and prurigo-nodularis, deviate from the classic flexural dermatitis.2 These variants are particularly resistant to conventional treatments including systemic immunosuppressive agents.3,4 Dupilumab has demonstrated excellent treatment response in adults with nummular eczema and prurigo-nodularis.3,4 Although the indication of dupilumab was extended from adult patients to paediatric atopic dermatitis patients,5 there is limited evidence regarding dupilumab effectiveness in paediatric patients with morphologic variants of atopic dermatitis. We present four paediatric cases with morphological variants of atopic dermatitis, who demonstrated dramatic improvement with dupilumab.
Overall four patients (aged 6–8 years, three males and one female) received dupilumab for atopic dermatitis between July 2020 and June 2021, in the dermatology clinic at Seoul National University of Bundang Hospital in Korea. The diagnosis of atopic dermatitis was confirmed by Hanifin and Rajka criteria and classified into specific morphologic phenotypes by an experienced dermatologist of the reference hospital.
Atopic dermatitis onsets for most patients were reported before the age of four months. All patients had generalised multiple eczematous lesions that were either refractory or minimally responsive to conventional treatments ranging from topical agents to systemic agents including cyclosporine and narrow-band ultraviolet-B therapy. Initial Eczema Area and Severity Index (EASI) of the subjects ranged from 17.1 to 24.7. Initial Investigator Global Assessment (IGA) scored 4 in all patients [Table 1]. All patients were treated with dupilumab at a starting dose of 600 mg subcutaneously followed by 300 mg every four weeks. All patients demonstrated remarkable clinical improvement after their first injection [Figures 1–4]. Based on IGA, all of our patients showed at least a 2-point reduction in score from baseline by week four and two patients achieved a score of zero by week 16. Based on EASI, all patients achieved at least 75% improvement (EASI–75) by week four. Most patients achieved IGA 0–1 by week 16 and these improvements were maintained over the next six months. Regarding adverse effects, only one patient reported mild conjunctivitis after initiation of dupilumab, which resolved with antihistamine ophthalmic solution.
| Patientno. | Age at DPLM initiation (y) | Gender | Onset of AD | Other atopic/ allergic conditions | Initial total IgE (IU/mL) | Phenotype of AD | EASI | IGA | Weight(kg) | Previous therapies | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wk 0 |
Wk 4 |
Wk 16 |
Wk 24 |
Wk 0 |
Wk 4 |
Wk 16 |
Wk 24 |
|||||||||
| 1 | 7 | M | 3 mo | Food allergy | >2500 | Nummular eczema | 24.7 | 2.8 | 1.2 | 0 | 4 | 2 | 1 | 0 | 20.0 | TCSs, TCIs, CsA |
| 2 | 6 | M | 4 mo | 98.85 | Prurigo nodularis | 21.6 | 2.9 | 0 | 0 | 4 | 2 | 0 | 0 | 19.0 | TCSs, TCIs, NB-UVB, CsA | |
| 3 | 8 | F | Infancy | NA | Prurigo nodularis | 17.1 | 2.3 | 0 | 0 | 4 | 1 | 0 | 0 | 26.0 | TCSs, TCIs | |
| 4 | 7 | M | 1 mo | Food allergy, allergic rhinitis | 20,000 | Prurigo nodularis | 20.2 | 4.8 | NA | NA | 4 | 1 | NA | NA | 22.0 | TCSs, TCIs, NB-UVB |
DPLM: Dupilumab, AD: Atopic dermatitis, y: Year, mo: Month, wk: Week, IgE: Immunoglobulin E, IGA: Investigator’s global assessment, EASI: Eczema area and severity index, TCS: Topical corticosteroid, TCI: Topical calcineurin inhibitor, NB-UVB: Narrow band ultraviolet B, CsA: Cyclosporine, NA: Not assessed
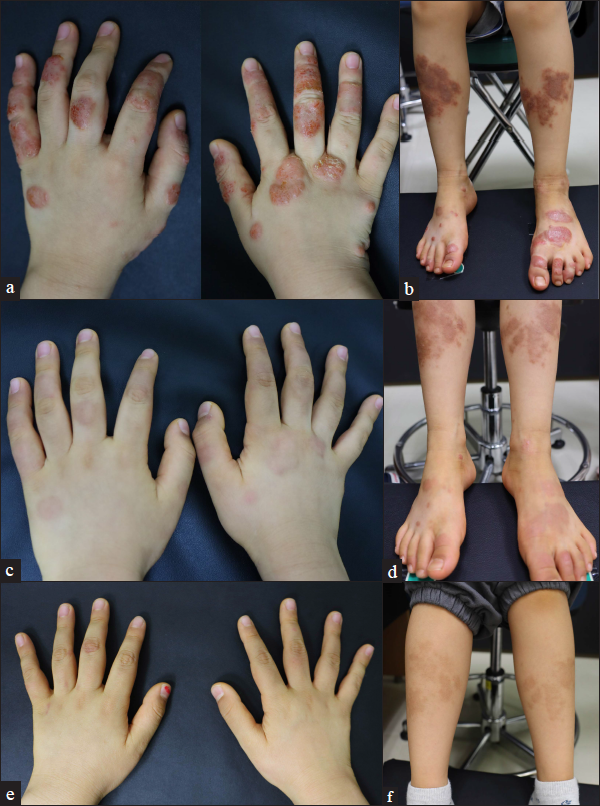
- Clinical images of patient 1 (atopic dermatitis with nummular eczema phenotype) at (a and b) baseline, (c and d) four weeks and (e and f) six months after their first injection of dupilumab
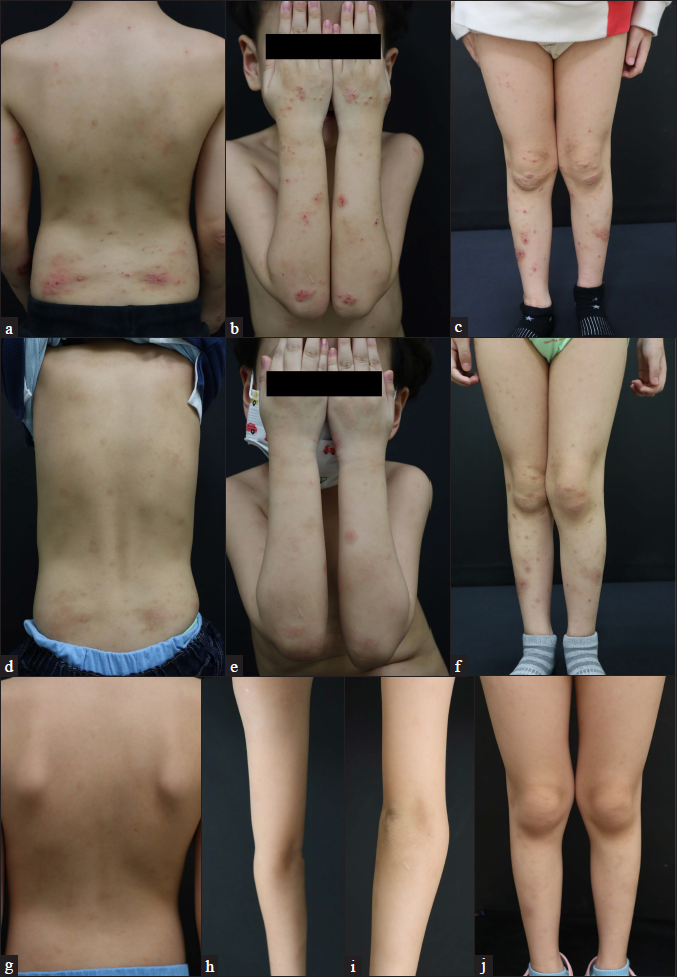
- Patient 2 (atopic dermatitis with prurigo nodularis phenotype) at (a–c) baseline, (d–f) four weeks and (g–j) six months after their first injection of dupilumab
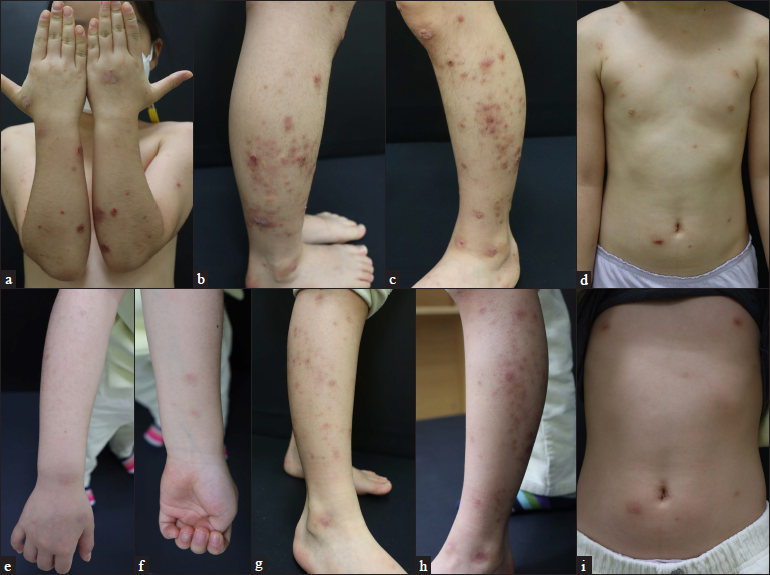
- Patient 3 (atopic dermatitis with prurigo nodularis phenotype) at (a–d) baseline and (e–i) four weeks after their first injection of dupilumab
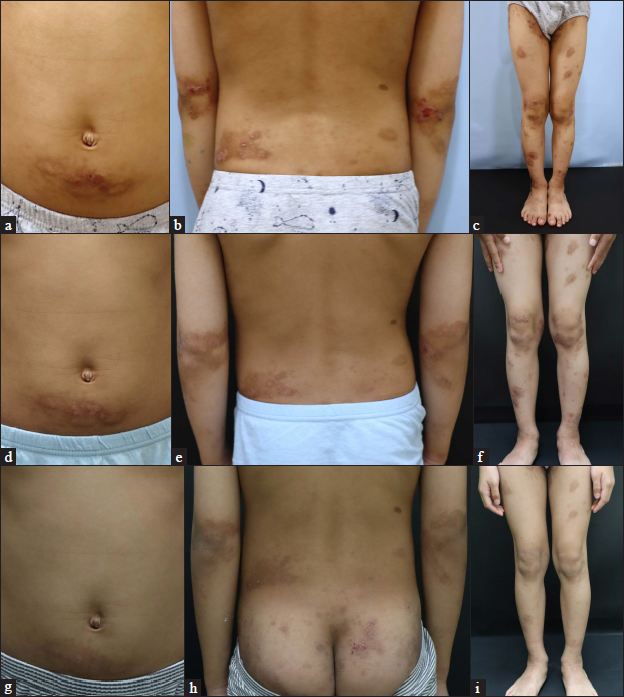
- Patient 4 (atopic dermatitis with prurigo nodularis phenotype) at (a–c) baseline, (d–f) four weeks and (g–i) six months after their first injection of dupilumab
The results from our dupilumab study in paediatric atopic dermatitis patients with nummular eczema and prurigo-nodularis phenotypes are consistent with previous findings in adult atopic dermatitis patients. In our resistant cases, dupilumab provided dramatic therapeutic responses as early as four weeks after initial administration. Our findings suggest that dupilumab can be an effective treatment not only for classic, typical atopic dermatitis but also for morphological variants of atopic dermatitis, especially in the understudied paediatric patient population.
All our cases who received dupilumab showed dramatic improvement in IGA or EASI score by week four, which were usually maintained over the next six months. These results are consistent with previous clinical studies in which atopic children (age 6–11 receiving dupilumab required only four weeks to achieve a significant improvement in EASI–75 response (P ≤ 0.0001) compared to the placebo group, whereas adult atopic dermatitis patients required 16 weeks to demonstrate a similar statistical difference.5,6 Another report highlighted a 5-year-old patient with severe atopic dermatitis achieving IGA score 1 only two weeks after dupilumab initiation.7 These findings support the conclusion that dupilumab may provide more rapid improvement in paediatric atopic dermatitis patients compared to adult atopic dermatitis patients.
This variable therapeutic timing may be attributed to differences in immune phenotypes between paediatric and adult atopic dermatitis patients. Based on previous findings, paediatric atopic dermatitis is characterised by Th2 and Th17 axis-skewing, while adult atopic dermatitis is characterised by Th2, Th22 and Th1 axis-skewing.8,9 Chronic atopic dermatitis lesions in adults have demonstrated increased Th1 axis activation parallel to intensified Th2 and Th22 responses,9 while acute or subacute atopic dermatitis lesions in childhood are characterised by a less activated Th1 axis. Additionally, dupilumab significantly reduced the expression of genes involved in type II inflammation and Th17/Th22 activity with no effect on Th1 gene expression.10 These differences in immune phenotypes may subject atopic dermatitis in early childhood to a more rapid response to dupilumab compared to adult atopic dermatitis. We were unable to find any previous reports demonstrating the clinical efficacy of dupilumab in morphological variants of atopic dermatitis in early childhood. In contrast to conventional treatments which often prove refractory in children with nummular eczema or prurigo-nodularis phenotypes, dupilumab remains effective in managing morphological variants of atopic dermatitis in early childhood.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Dupilumab for off-label treatment of moderate to severe childhood atopic dermatitis. Cutis. 2018;102:201-4.
- [PubMed] [Google Scholar]
- Phenotypes of atopic dermatitis. J Dtsch Dermatol Ges. 2011;9:12-20.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab for prurigo nodularis: Case series and review of the literature. Dermatol Ther. 2020;33:e13222.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of dupilumab for the treatment of generalized prurigo nodularis phenotype of adult atopic dermatitis. Dermatitis. 2020;31:81-4.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: A randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-93.
- [CrossRef] [PubMed] [Google Scholar]
- Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-48.
- [CrossRef] [PubMed] [Google Scholar]
- Remarkable response to dupilumab in a 5-year-old patient with severe, recalcitrant atopic dermatitis. JAAD Case Rep. 2019;5:605-8.
- [CrossRef] [PubMed] [Google Scholar]
- Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J Allergy Clin Immunol. 2016;138:1639-51.
- [CrossRef] [PubMed] [Google Scholar]
- Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344-54.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143:155-72.
- [CrossRef] [PubMed] [Google Scholar]





