Translate this page into:
Concentration of substance P in patients with atopic dermatitis with and without past history of treatment
Corresponding author: Dr. Nova Zairina Lubis, Department of Dermatology and Venereology, Universitas Sumatera Utara Hospital, Universitas Sumatera Utara, Medan, Indonesia. novazai82@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Lubis NZ, Nasution K, Paramita DA. Concentration of substance P in patients with atopic dermatitis with and without past history of treatment. Indian J Dermatol Venereol Leprol 2023;89:458-9.
Dear Editor,
Atopic dermatitis is a chronic, inflammatory skin disease that initially appears in childhood. Itching is the primary symptom in atopic dermatitis patients. It causes sleep disturbances and skin infections.1 The prevalence rate of atopic dermatitis has proliferated in the last decade, that is, 10–20% cases in infants and children and around 1–3% in adults. Atopic dermatitis was present in about 1.1% of patients aged 13–14 years in 2012.2 The International Study of Asthma and Allergies in Childhood found that the morbidity rate reached 20% in Asian countries, including South Korea, Taiwan and Japan. In developing countries, an estimated 10–20% of children suffer from atopic dermatitis. Additionally, 60% of atopic dermatitis patients live until adulthood.3
The pathogenesis of atopic dermatitis is poorly understood. Skin neuropeptides, particularly substance P, contribute to the pathogenesis of various skin diseases, such as atopic dermatitis. Substance P promotes nerve growth factors from keratinocytes, histamine release, leukotriene or tumour necrosis factor from mast cells. This condition causes the growth of sensory nerve fibres and augmentation of skin inflammation. Therefore, substance P is currently considered a pruritogenic factor.4 Substance P induces an itching response in humans and mice and is mediated through the activation of the neurokinin 1 receptor on mast cells and keratinocytes. Moreover, it causes an increased inflammatory response and supports substance P’s indirect effect in mediating pruritus.5 Several studies have shown that blocking itching signals through neurokinin 1 receptor reduces itching complaints.6
This study aimed to evaluate the relationship between treatment history and substance P levels in atopic dermatitis in children.
This was a cross-sectional study conducted from February to June 2020 at the outpatient Department of Dermatology and Venereology, Division of Pediatric and Adolescent Dermatology at Universitas Sumatera Utara in Medan, Indonesia. Blood samples were analysed at the Integrated Laboratory.
Female and male patients with atopic dermatitis were enrolled in this study based on Hanifin and Rajka’s criteria. All participants who were willing to participate had signed informed consents. The exclusion criteria were: participants who had any other skin disease or a systemic disease and patients whose parents were unable to answer the questions.
Blood samples were obtained to measure the substance P levels using an ELISA kit (R&D Systems) according to the manufacturer’s instructions. Determination of the optical density of each sample was conducted in 30 min using a microplate reader set at 450 nm. The results were presented in units of pg/mL. This study was conducted in accordance with the Declaration of Helsinki.
A total of 46 participants with atopic dermatitis, 29 males (63%) and 17 females (37%) [Table 1] were enrolled in this study. The mean age of subjects with atopic dermatitis was 10.35 years (standard deviation = 4.01), with the youngest being 1 year and the oldest being 17 years old [Table 1].
| Demographic | n = 46 (%) |
|---|---|
|
Gender Male Female Old (years) Mean Standard deviation (SD) Median Min-max Medication history Yes No Substance P levels Mean Standard deviation (SD) Median Min-max |
29 (63%) 17 (37%) 10.35 4.01 11 1–17 17 (37%) 29 (63%) 300.88 127.55 270.50 172.4–764.4 |
Based on treatment history, 17 subjects (37%) had a history of atopic dermatitis treatment, while 29 subjects (63%) had never received or had no history of atopic dermatitis treatment [Table 1]. The mean value of substance P levels from 46 atopic dermatitis patients was 300.88 (standard deviation = 127.55) with the lowest level of 172.4 pg/mL and the highest level of 764.4 pg/mL [Table 1].
There was no significant relationship between the history of atopic dermatitis treatment and the level of substance P based on the Mann–Whitney U test (P = 0.733) [Table 2]. Subjects with a history of atopic dermatitis treatment had a lower median value of 262.8 (range, 172.4–764.4), whereas subjects without a history of atopic dermatitis treatment had a median value of 275.2 (range, 172.4–546.8) [Figure 1].
| Medication history | n | P level median (min–max) | P-value |
|---|---|---|---|
| Yes | 17 | 262.8 (172.4–764.4) | 0.733 |
| No | 29 | 275.2 (172.4–546.8) |
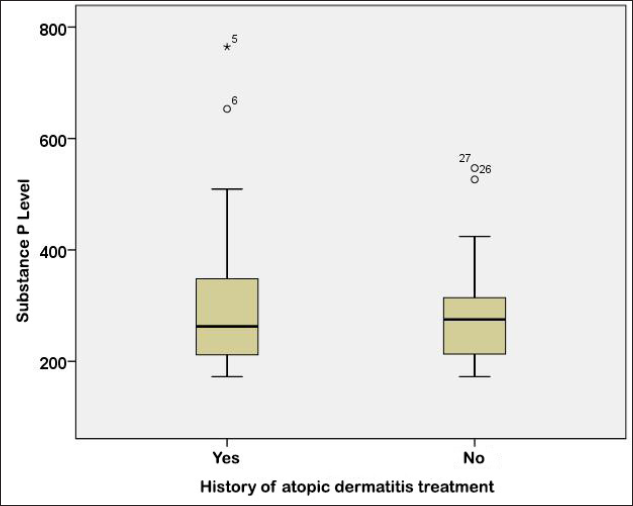
- Boxplot graph of substance P level based on the history of atopic dermatitis treatment
Several inflammatory neuropeptides play a vital role in the pathophysiology of pruritus in atopic dermatitis, especially the substance P and the neurokinin 1 receptor. Substance P levels are associated with atopic dermatitis disease activity and increased pruritus symptoms.7 In atopic dermatitis, there were specific neurobiological changes, due to stress. Stress has the adjunct effect with the increase of substance P, which can increase the production of vascular cell adhesion, skin thickness and vascular eosinophilic infiltration parameters.8 Although there is no significant relationship between the treatment history and the substance P levels in our study, the median level of substance P levels was lower in subjects with a history of atopic dermatitis treatment and higher in subjects without atopic dermatitis treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Research Foundation of Universitas Sumatera Utara Budget Period of 2020 supported this research with the Letter of Agreement for Research Implementation of the Mono Program for Basic Research in the fiscal year 2020 Number 62/UN5.2.3.1/PPM/SPP-TALENTA USU/2020 dated April 28th 2020
Conflict of interest
There are no conflicts of interest.
References
- Atopic Dermatitis In: Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael A, eds. Fitzpatrick’s Dermatology in General Medicine (9th ed.). New York: Mc Graw Hill; 2019. p. :363-381.
- [Google Scholar]
- Panduan Diagnosis dan Tatalaksana Dermatitis Atopik di Indonesia [Diagnosis and Management Guidelines of Atopic Dermatitis in Indonesia] Jakarta: Centra Communications; 2014. p. :1-2.
- [Google Scholar]
- Atopic eczema in children: NICE quality standard. Community Pract. 2013;86:46.
- [PubMed] [Google Scholar]
- Severity scores, itch scores and plasma substance P levels in atopic dermatitis treated with standard topical therapy with oral olopatadine hydrochloride. J Dermatol. 2009;36:185-90.
- [CrossRef] [PubMed] [Google Scholar]
- Itch in atopic dermatitis - Pathophysiology and treatment. Acta Dermatovenerol Croat. 2010;18:289-96.
- [PubMed] [Google Scholar]
- Substance P (SP) and Neurokinin 1 Receptor (NK1R) are new targets for the treatment of chronic pruritus. Br J Dermatol. 2019;181:932-8.
- [CrossRef] [PubMed] [Google Scholar]
- Current pharmaceutical developments in atopic dermatitis. Curr Opin Pharmacol. 2019;46:7-13.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic dermatitis: Allergic dermatitis or neuroimmune dermatitis? An Bras Dermatol. 2016;91:479-88.
- [CrossRef] [PubMed] [Google Scholar]





