Translate this page into:
Clinical and metabolic characteristics of males with early-onset androgenetic alopecia
Corresponding author: Dr. Keshavamurthy Vinay, Departments of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Sector 12, Chandigarh, India. vinay.keshavmurthy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vinay K, Bhattachajee R, Bishnoi A, Kaushik A, Sachdeva N, Pal A, et al. Clinical and metabolic characteristics of males with early-onset androgenetic alopecia. Indian J Dermatol Venereol Leprol 2023;89:530–5.
Abstract
Background
Men with early-onset androgenetic alopecia (AGA) often have an abnormal hormonal milieu.
Objective
To ascertain the clinico-phenotypic characteristics and the prevalence of hormonal and metabolic changes in men with early-onset AGA.
Methods
Consecutive male patients less than 30 years of age with a Norwood-Hamilton grade ≥3 AGA were recruited in this comparative cross-sectional study. After endocrine evaluation they were classified into two groups, that is, Group A consisting of subjects with an altered hormonal profile and Group B with normal hormonal profiles. The groups were assessed for differences in disease phenotype and severity (Norwood-Hamilton grade), insulin resistance and parameters of metabolic syndrome (ATP III guidelines).
Results
Altered hormonal profiles were seen in 34 of the 100 subjects with AGA, while insulin resistance and metabolic syndrome were noted in 44 and 26 respectively. Altered hormonal profiles were significantly associated with insulin resistance and severe alopecia (grade 4 and above Hamilton-Norwood Scale). Insulin resistant Group A patients had a significantly higher prevalence of severe alopecia (>grade 4) (P = 0.0036). The prevalence of metabolic syndrome was similar in both groups.
Limitation
The cross sectional study design was a drawback of this study. Further, a control arm without AGA was not included and the sample size of 100 was selected arbitrarily.
Conclusion
An altered hormonal profile and insulin resistance was noted in a third of the males with early-onset AGA. Subjects with altered hormonal profiles had a higher prevalence of insulin resistance and were likely to have severe grades of AGA.
Keywords
Androgenetic alopecia
early onset
hormonal disturbance
insulin resistance
male androgenetic alopecia
metabolic syndrome
Plain Language Summary
Men with baldness frequently show hormonal disturbances similar to those seen in females with polycystic ovarian syndrome. This study was conducted at PGIMER, Chandigarh in men with moderate to severe baldness starting before the age of 30 years. Clinical features and prevalence of hormonal or metabolic changes were assessed. Patients were classified into two groups based on the endocrine evaluation—Group A with hormonal changes and Group B with normal hormonal profiles. Both groups were then compared with each other for differences in clinical features and severity of baldness, and presence of metabolic abnormalities. Of the 100 study patients, 34 had an abnormal hormonal profile, 44 had insulin resistance and 26 had metabolic syndrome. Patients with abnormal hormonal profiles also had more severe baldness. Patients with both insulin resistance and hormonal changes had higher grades of baldness than either alone. Our study shows that early-onset baldness (<30 years) is often associated with hormonal disturbances and insulin resistance. Patients with hormonal disturbances and insulin resistance are more likely to have higher grades of baldness compared to men with normal hormonal profiles and no metabolic disturbances.
Introduction
Androgenetic alopecia (AGA) is a common androgen-dependent progressive loss of hair primarily affecting the scalp hair in males. Females are sometimes afflicted, but the pattern of hair loss is often distinct from that in males. While the majority of males with this condition present after the age of thirty, some present earlier during adolescence or in the early twenties. The term early-onset AGA is used for such patients, but the exact cut off is defined variably in literature as either 30 or 35 years.1,2
Polycystic ovarian syndrome (PCOS) is an endocrine disorder characterised by chronic hyperestrogenism, hyperandrogenism, anovulation, infertility, obesity, insulin resistance and cardiovascular problems.3 Early-onset AGA is known to occur in first degree male relatives of females with PCOS and these men are also at increased risk of insulin resistance, obesity, diabetes mellitus and cardiovascular disease, similar to females with PCOS.4,5 Given the genetic basis of PCOS, it has been suggested that early-onset AGA in men could be the phenotypic equivalent of PCOS in females.6-8
The aim of this study was to ascertain the prevalence of hormonal abnormalities in males with early-onset AGA. We also wished to compare the incidence of insulin resistance and metabolic syndrome in patients with early-onset AGA with normal hormonal profiles with those with abnormal profiles.
Methods [Figure 1]
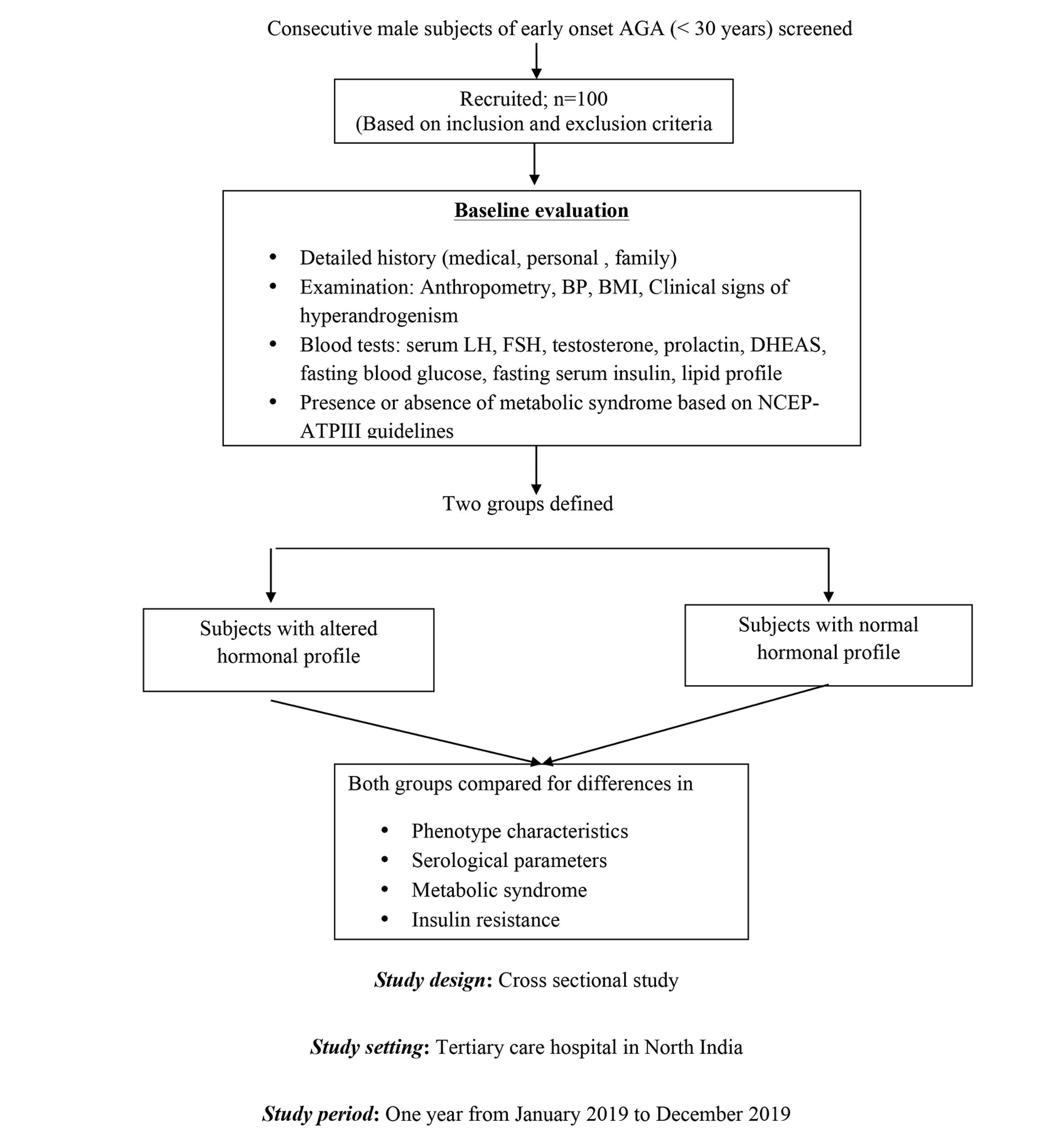
- Study design. AGA: Androgenetic alopecia, BP: Blood pressure, BMI: Body mass index, DHEAS: Dehydroepiandrosterone sulfate, FSH: Follicle stimulating hormone, LH: Luteinizing hormone
Study subjects
This comparative cross-sectional study was conducted at the dermatology outpatient clinic of the Postgraduate Institute of Medical Education & Research, Chandigarh from January to December 2019. Consecutive consenting male patients with early-onset AGA (aged below 30 years) with a Norwood-Hamilton grade ≥3 were included in this study. The study was approved by the Institute Ethics Committee and was registered in the Clinical Trial Registry - India (CTRI/2018/11/016269).
Subjects with diabetes, hypertension, hepatic or renal disorders and chronic infections (e.g., tuberculosis, human immunodeficiency virus, hepatitis B and C) were excluded from the study. We also excluded patients with alopecia due to other established causes and those who had received androgen supplements, hormonal therapy or finasteride.
Baseline evaluation
A detailed medical, personal and family history was recorded for each subject. Anthropometric measurements, blood pressure, the body mass index and the pattern and Norwood-Hamilton grade of alopecia were recorded.9 Clinical signs of hyperandrogenism (e.g., seborrhoea and acne) and the presence of metabolic syndrome (diagnosed as per criteria of the modified National Cholesterol Education Program’s Adult Treatment Panel III (NCEP ATP III)) were noted.10
Investigations were performed in all cases including fasting blood glucose, fasting serum insulin, lipid profile, serum luteinizing hormone (LH), serum follicle-stimulating hormone (FSH), serum testosterone (total and free), sex hormone-binding globulin (SHBG), serum prolactin, serum dehydroepiandrosterone sulfate (DHEAS). The reference levels of sex hormones were defined in accordance with the ranges described in an earlier study of a male population of similar ethnicity and region.1 The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using a standard formula (fasting insulin (microunits/mL) × fasting glucose (micrograms/dL)/405) and a value greater than 2.5 was defined as insulin resistance.
Categorisation of study subjects
An altered hormonal profile was defined as two or more of the following:7,8
SHBG <15 nmol/l
free androgen index (total testosterone/ SHBG x 100) >150
FSH <1 mIU/mL or luteinizing hormone:follicle stimulating hormone ratio >1.0
DHEAS levels >3.6 μg/mL.
Those with an altered hormonal profile were classified as Group A and the rest (i.e., with a normal hormonal profile) as Group B. This categorization was based entirely on laboratory findings and no clinical features were taken into consideration.
Both groups were then compared for differences in dermatological features suggesting hyperandrogenism and insulin resistance (pattern and severity of AGA, facial and truncal acne, seborrhoea, acanthosis nigricans and skin tags) and biochemical and physical parameters relating to metabolic syndrome (blood glucose, serum insulin levels, insulin resistance, lipid profile, body mass index, blood pressure and waist circumference) using appropriate statistical tests. The severity of AGA was graded as mild (Norwood-Hamilton grade 3) or severe (Norwood-Hamilton grade 4 and above).11 Sub-analyses were then performed after categorizing the subjects into four groups based on the presence or absence of either an altered hormonal profile or insulin resistance or both.
Statistical analysis
All statistical calculations were made using a statistical package for the social sciences software (SPSS v. 19). Data were summarized using mean ± standard deviation for quantitative variables and percentages for qualitative variables. Comparisons between the groups were made using the Student t-test for quantitative variables, Pearson’s chi-square analysis for qualitative variables, logistic regression for analysis of correlation between groups and individual parameters. All statistical values with P < 0.05 were taken as statistically significant.
Results
Patients
A total of 100 male subjects with AGA were recruited in the study. The mean age was 24.12 ± 2.0 years (range 18-29 years) and mean disease duration of 2.1 ± 1.8 years. A family history of patterned hair loss in first-degree male relatives was present in 50 patients and a history of PCOS in first-degree female relatives was seen in 34 study subjects. Altered hormonal profiles were noted in 34 patients (Group A) and the remaining were categorized as Group B [Table 1].
| Group A (Altered hormonal profile, n = 34) | Group B (Normal hormonal profile, n = 66) | P-value | |
|---|---|---|---|
| Demographic parameters | |||
| Age in years (Mean ± SD) | 24.12 ± 1.2 | 23.97 ± 1.5 | 0.922 |
| Mean duration of disease (years) | 2.1 ± 1.4 | 2.2 ± 1.7 | 0.954 |
| Positive family history of early onset AGA or PCOS | 22 (64.7%) | 34 (51.5%) | 0.704 |
| Treatment history | Minoxidil, n = 20 Biotin, n = 2 | Minoxidil, n = 16 Biotin, n = 0 | - |
| Clinical evaluation | |||
| Mean body mass index (kg/m2) | 24.474 ± 2.76 | 22.688 ± 3.11 | 0.605 |
| Mean systolic blood pressure (mm Hg) | 132 ± 12 | 128 ± 14 | 0.788 |
| Mean diastolic blood pressure (mm Hg) | 82 ± 10 | 80 ± 14 | 0.884 |
| Mean waist circumference (in cm) | 82.5 ± 7.22 | 85.5 ± 6.40 | 0.703 |
| Acne vulgaris | 8 (23.5%) | 13 (19.7%) | 0.655 |
| Mild acne vulgaris | 3 (8.8%) | 6 (9.1%) | 0.964 |
| Moderate acne vulgaris | 5 (14.7%) | 7 (10.6%) | 0.550 |
| Seborrhoea | 8 (23.5%) | 14 (21.2%) | 0.791 |
| Acanthosis nigricans | 3 (8.8%) | 1 (1.5%) | - |
| Skin tags | 1 (2.9%) | 1 (1.5%) | - |
| Patterns of alopecia | |||
| A. Predominantly temporal recession | |||
| i. Temporal alone | 3 (8.8%) | 6 (9.1%) | 0.242 |
| ii. Temporal with vertex | 12 (35.3%) | 22 (33.3%) | |
| iii. Temporal with frontal | 10 (29.4%) | 24 (36.3%) | |
| iv. Temporal with frontal and vertex | 2 (5.9%) | 5 (7.5%) | |
| B. Other patternsa | 5 (14.7%) | 8 (12.1%) | |
| C. Diffuse hair loss | 2 (5.9%) | 1 (1.5%) | |
| Subjects with grade ≥4 Hamilton and Norwood | 13 (38.2%) | 11 (16.7%) | 0.016 |
a: Includes frontal recession, vertex involvement or both, with minimal temporal recession
Demographic and clinical features
The two groups were similar with respect to age, disease duration, family history, the presence of seborrhoeic dermatitis and acne vulgaris. A significantly higher proportion of subjects in Group A had alopecia of grade 4 and above (41.1% vs 16.7%, P = 0.007). Temporal recession with vertex or frontal involvement was the most common pattern in both groups.
Endocrine evaluation and insulin resistance [Table 2]
| Group A (Altered hormonal profile, n = 34) | Group B (Normal hormonal profile, n = 66) | P-value | |
|---|---|---|---|
| Biochemical evaluation | |||
| Mean total triglycerides (mg/dL) | 169. 18 ± 31.5 | 164.75 ± 43.2 | 0.917 |
| Mean high density lipoprotein (mg/dL) | 44.95 ± 4.66 | 42.80 ± 10.22 | 0.812 |
| Mean fasting blood glucose (mg/dL) | 98.85 ± 10.22 | 92.16 ± 12.23 | 0.613 |
| Mean fasting serum insulin | 22.51 ± 3.78 | 21.44 ± 4.55 | 0.822 |
| Mean HOMA-IR | 5.54 ± 5.75 | 3.37 ± 4.17 | 0.033 |
| Insulin resistance | 22 (66.7%) | 22 (33.3%) | 0.003 |
| Metabolic syndrome | 8 (23.5%) | 18 (27.2%) | 0.686 |
| Endocrine evaluation | |||
| Mean follicle stimulating hormone levels (mIU/mL) | 2.01 ± 1.28 | 2.84 ± 1.12 | 0.0012 |
| Mean prolactin values (ng/mL) Mean luteinizing hormone levels (mIU/mL) | 16.3 ± 2.1 6.59 ± 2.33 | 15.81 ± 3.4 5.88 ± 3.44 | 0.44 0.2823 |
| Mean serum testosterone (nmol/L) | 17.73 ± 10.22 | 15.74 ± 8.99 | 0.3195 |
| Mean DHEAS (μg/dL) | 367.3 ± 54.3 | 334 ± 68.32 | 0.0154 |
| Male polycystic ovarian syndrome equivalent7,8 (Fisher’s Exact Test) | |||
| Sex hormone binding globulin (<15 nmol/l) | 14 (41.1%) | 10 (15.1%) | 0.006 |
| Follicle stimulating hormone (<1 mIU/mL) | 12 (35.3%) | 9 (13.6%) | 0.018 |
| Free androgen index (>150) | 10 (29.4%) | 8 (12.1%) | 0.0524 |
| DHEAS levels (>3.6 μg/mL) | 15 (44.1%) | 9 (13.6%) | 0.0012 |
DHEAS, dehydroepiandrosterone sulfate; HOMA-IR, homeostatic model assessment-insulin resistance
All four criteria for an abnormal hormonal profile were fulfilled in eight of the 34 patients in Group A while either 2 or 3 criteria were present in the remainder. Twenty-two subjects in each group had insulin resistance but the proportion of patients with insulin resistance was significantly greater in Group A (66.7% vs 33.3%, P = 0.003). Although the mean fasting serum insulin levels were similar in both groups (P = 0.822) the mean homeostatic model assessment for insulin resistance values was significantly greater in Group A (P = 0.033). A higher prevalence of severe alopecia (grade 4 and above) was noted in subjects with insulin resistance but the difference was not significant (P = 0.64).
Metabolic syndrome
The prevalence of metabolic syndrome was similar in both groups (26% of all subjects; 23.5% in Group A and 27.2% in Group B, P = 0.686) as were the individual parameters (i.e., body mass index, blood pressure, mean waist circumference, total triglycerides, mean high-density lipoprotein and mean fasting plasma glucose values).
Sub-group analyses [Table 3]
| AHP + IR+ (n = 15) | AHP + IR- (n = 19) | AHP-IR+ (n = 16) | AHP-IR- (n = 50) | P-value | |
|---|---|---|---|---|---|
| Age in years (Mean ± SD) | 23.75 ± 1.2 | 23.56 ±2.4 | 23.87 ± 1.5 | 24.1 ± 1.6 | 0.42 |
| Mean duration of disease (years) | 2.05 ± 1.7 | 2.19 ± 2.1 | 2.2 ± 1.7 | 2.3 ±1.6 | 0.843 |
| Subjects with grade ≥4 Hamilton and Norwood | 10 (45.5%) | 3 (25%) | 2 (9%) | 9 (20.5%) | 0.036 |
| Metabolic syndrome | 7 (31.8%) | 1 (8.33%) | 6 (27.3%) | 12 (27.3%) | 0.495 |
| Mean total triglycerides (mg/dL) | 165.39 ± 32.75 | 169.21 ± 27.33 | 168.76 ± 34.87 | 166.65 ± 41.63 | 0.913 |
| Mean high density lipoprotein (mg/dL) | 48.10 ± 8.45 | 42.76 ± 6.22 | 43.75 ± 14.23 | 42.87 ± 10.45 | 0.616 |
| Mean fasting blood glucose (mg/dL) | 102.3 ± 19.25 | 93.54 ± 14.21 | 102.66 ± 18.34 | 91.18 ± 14.23 | 0.018 |
| Mean fasting serum insulin | 28.22 ± 3.11 | 21.56 ± 3.12 | 22.46 ± 4.55 | 21.45 ± 4.34 | 0.0045 |
| Mean HOMA-IR | 7.43 ± 6.16 | 1.56 ± 0.57 | 7.36 ± 5.31 | 1.38 ± 0.56 | 0.001 |
| Mean follicle stimulating hormone levels (mIU/mL) | 3.11 ± 1.18 | 2.2 ± 1.14 | 2.17 ± 2.1 | 2.18 ± 1.77 | 0.04 |
| Mean luteinizing hormone levels (mIU/mL) | 6.74 ± 4.12 | 6.14 ± 1.98 | 5.88 ± 3.18 | 5.66 ± 2.22 | 0.877 |
| Mean serum testosterone (nmol/l) | 16.86 ± 9.05 | 16.8 ± 10.1 | 15.96 ± 8.12 | 15.87 ± 7.98 | 0.624 |
| Mean DHEAS (μg/dL) | 401.5 ± 70.6 | 356.78 ± 63.21 | 364.56 ± 51.2 | 321.5 ± 79.21 | 0.0034 |
AHP: abnormal hormonal profile, DHEAS: dehydroepiandrosterone sulfate, HOMA-IR: homeostatic model assessment-insulin resistance, IR: Insulin resistance
Sub-group analyses revealed that patients with both altered hormonal profiles and insulin resistance had a significantly higher prevalence of severe alopecia (grade 4 and above; P = 0.036), significantly higher mean DHEAS levels (P = 0.002) and significantly lower mean FSH (P = 0.021). The prevalence of metabolic syndrome was similar across the groups (P = 0.066).
Discussion
A complex interplay of hormonal, genetic and inflammatory factors resulting in miniaturization of hair follicles underlies the hair loss in AGA. Premature or early-onset of AGA (before 30 years of age) occurs in about 30% of men and endocrinological findings in such patients are well documented in literature.7,12-16 However, phenotypic comparisons in premature AGA patients with and without hormonal abnormalities have not been reported.
Previous studies of sex related hormones (FSH, LH, SHBG, free androgen index and DHEAS) in early AGA have given conflicting results and the evidence for hyperandrogenism has been variable.1,7,15,16 Sanke et al. reported elevated levels of testosterone, prolactin and LH in early-onset AGA and noted similarities with PCOS.1 Decreased levels of SHBG and a high free androgen index in men with early-onset AGA compared to healthy controls have also been described.16 We noted high DHEAS and low total testosterone levels in our study and similar results were recently reported by Cannarella et al. in early-onset AGA.7
Insulin resistance was present in 44% of our patients with early-onset AGA while Bakry et al. reported this in 35% of their patients with AGA.17 Insulin resistance was particularly prevalent in the patients with altered hormonal profiles (66.7% vs. 33.3%) but it was not associated with the severity of AGA. We did not find any studies directly correlating AGA severity with insulin resistance, but we observed a significantly greater prevalence of severe alopecia in patients who had both altered hormonal profiles and insulin resistance.
There have been conflicting reports regarding the association of early onset AGA and metabolic syndrome.18,19 Although we found a significantly higher proportion of insulin resistance in patients with altered hormonal profiles, the prevalence of metabolic syndrome was similar in both groups. Insulin resistance often predates the development of clinically apparent metabolic syndrome and such patients should be carefully observed for the development of metabolic syndrome in the future.
Future implications
Our findings suggest an association of hormonal imbalance and insulin resistance with the severity of early-onset AGA. Insulin resistance may play a role in the pathogenesis and progression of AGA and future studies should assess its role in the progression/reversal of AGA. Since insulin resistance is associated with cardiovascular risk factors, this subset of patients with early-onset AGA should be identified so as to incorporate corrective lifestyle changes in order to prevent future cardiovascular morbidity.
Limitations
The cross-sectional nature of the study was one of the limitations of our study since causal association could not be determined. Since a control arm without AGA was not included, formal sample size estimation was not possible. A convenient sample size of 100 was chosen for this study.
Conclusion
Altered hormonal profile and insulin resistance are common in men with early-onset AGA, seen in up to one-third of such subjects. The severity of AGA is greater when both altered hormonal profile and insulin resistance occur together.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
This study was funded by the IADVL research grants.
Conflicts of interest
There are no conflicts of interest.
References
- A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatology. 2016;152:986-91.
- [CrossRef] [PubMed] [Google Scholar]
- The existence of a male equivalent of the polycystic ovary syndrome-the present state of the issue. Prague Med Rep. 2006;107:17-25.
- [PubMed] [Google Scholar]
- Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27-36.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of metabolic syndrome in the family members of women with polycystic ovary syndrome from North India. Indian J Endocrinol Metab. 2014;18:364-9.
- [CrossRef] [PubMed] [Google Scholar]
- Glucose intolerance, insulin resistance and cardiovascular risk factors in first degree relatives of women with polycystic ovary syndrome. Hum Reprod. 2005;20:2414-20.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic basis of polycystic ovary syndrome (PCOS): Current perspectives. Appl Clin Genet. 2019;12:249-60.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Increased DHEAS and decreased total testosterone serum levels in a subset of men with early-onset androgenetic alopecia: Does a male PCOS-equivalent exist? Int J Endocrinol. 2020;2020:1942126.
- [CrossRef] [PubMed] [Google Scholar]
- Does a male polycystic ovarian syndrome equivalent exist? J Endocrinol Invest. 2018;41:49-57.
- [CrossRef] [PubMed] [Google Scholar]
- Male pattern baldness: Classification and incidence. South Med J. 1975;68:1359-65.
- [CrossRef] [PubMed] [Google Scholar]
- Executive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486-97.
- [CrossRef] [PubMed] [Google Scholar]
- Male pattern androgenetic alopecia in an Indian context: A perspective study. J Eur Acad Dermatol Venereol. 2007;21:473-9.
- [CrossRef] [PubMed] [Google Scholar]
- Androgenetic alopecia in men and women. Clin Dermatol. 2001;19(2):167-78.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology,pathogenesis, genetics & management of polycystic ovary syndrome in India. Indian J Med Res. 2019;150:333-44.
- [CrossRef] [PubMed] [Google Scholar]
- Sex steroids and insulin resistance. Clin Sci. 2002;102:151-66.
- [CrossRef] [PubMed] [Google Scholar]
- Hormonal profile of men with premature balding. Exp Clin Endocrinol Diabetes. 2004;112:24-8.
- [CrossRef] [PubMed] [Google Scholar]
- Hormonal profile in Indian men with premature androgenetic alopecia. Int J Trichology. 2013;5:69-72.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Androgenetic alopecia metabolic syndrome, and insulin resistance: Is there any association? A case-control study. Indian Dermatol Online J. 2014;5:276-81.
- [CrossRef] [PubMed] [Google Scholar]
- The Association of Metabolic Syndrome and Insulin Resistance in early-onset androgenetic alopecia in males: A case-control study. Indian J Dermatol. 2019;64:23-7.
- [CrossRef] [PubMed] [Google Scholar]
- The investigation of insulin resistance and metabolic syndrome in male patients with early-onset androgenetic alopecia. Eur J Dermatology. 2011;21:79-82.
- [CrossRef] [PubMed] [Google Scholar]






