Translate this page into:
Efficacy and safety of 250 mg versus 500 mg oral terbinafine in the treatment of tinea corporis and cruris: A randomised, assessor-blinded comparative study
Corresponding author: Dr. Abanti Saha, Associate Professor, Department of Dermatology, Medical College, Kolkata, West Bengal, India. sahaaby@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Lal NR, Basu D, Saha A, Ghosh R, Verma R, Bandyopadhyay D. Efficacy and safety of 250 mg versus 500 mg oral terbinafine in the treatment of tinea corporis and cruris: A randomised, assessor-blinded comparative study. Indian J Dermatol Venereol Leprol 2023;89:665-71
Abstract
Background
Though higher doses of terbinafine are often prescribed to treat dermatophyte infections, it is unknown if such doses are more effective than the conventional dose because comparative data are unavailable.
Aim
To compare the efficacy and safety of a once-daily dose of oral terbinafine 250 mg with 500 mg along with topical clotrimazole in the treatment of tinea infections.
Methods
A randomised, assessor-blinded, comparative study was carried out. Each group of subjects were administered either 250 mg or 500 mg oral terbinafine once daily for four weeks, along with topical clotrimazole. Clinical improvement was assessed after two weeks and again after four weeks from treatment initiation.
Result
A total of 60 patients with tinea corporis and cruris were randomised into two groups receiving either 250 mg (group A) or 500 mg (group B) oral terbinafine, along with clotrimazole cream in both groups. Baseline clinical parameters such as lesional activity (papules, vesicles and pustules), degree of erythema, scaling and severity of itching were comparable between both treatment arms. At the first and second follow-ups, no significant differences were found in the clinical parameters between the two groups. At the end of two weeks 13.8% of group A and 14.3% of group B and after 4 weeks 25.9% of group A and 33.3% of group B participants became KOH negative (P = 1.00 and 0.76, respectively). No significant difference in culture negativity was reported at the end of therapy (four weeks) between the two treatment arms (P = 0.78). Overall cure rates were 20% and 33.3% in the two treatment arms respectively at the end of the study (P = 0.82).
Conclusion
Oral terbinafine 250 mg daily yielded a poor cure rate in tinea cruris and corporis after 4 weeks of treatment and an increased dose of 500 mg did not have any additional benefit.
Keywords
Tinea
terbinafine
oral
assessor-blinded
randomised clinical trial
Plain Language Summary
Tinea corporis and cruris refer to ringworm infections of the body and groin, respectively. The current situation of ringworm in India is quite alarming as many patients are having widespread, long-lasting and recurrent disease and the infection is no longer responding to conventional dosage and duration of treatment. To combat this situation, oral antifungal drugs are often being used at higher doses and for longer durations. We undertook a randomised, assessor-blinded, comparative study to see whether doubling the usual dosage of terbinafine, a drug of choice for ringworm infection, from 250 mg/day to 500 mg/day has a better effect. We recruited 60 patients who were randomly treated with either 250 mg (group A) or 500 mg (group B) oral terbinafine, along with clotrimazole cream in both groups, for four weeks. At the end of the study, the overall cure rates were 20% in group A and 33.3% in group B, a difference that was not statistically significant. We concluded that oral terbinafine 250 mg daily yielded a poor cure rate in tinea cruris and corporis after 4 weeks of treatment and an increased dose of 500 mg did not have any additional benefit.
Introduction
Dermatophyte infections are superficial fungal infections of skin, hair and nails. Commonly known as ringworm, these infections are caused by species of genera Trichophyton, Microsporum and Epidermophyton.1 Tinea corporis and cruris refer to dermatophyte infection of glabrous skin and groin, respectively.1 Though not life-threatening, they can cause considerable discomfort in affected patients.
The current state of dermatophytosis in India is quite alarming, with patients presenting with extensive involvement and chronic recurrent infections. More importantly, these infections no longer respond to conventional dosages and durations of therapy. To combat this situation, dermatologists are using various combinations of oral antifungals, often at higher doses, for longer durations, and even adding retinoids in the management of recalcitrant tinea cases.2 Of late, dermatologists have been upscaling the dose of oral terbinafine to 500 mg daily either as a single daily dose or in divided doses. There are quite a few studies which have explored and reported 500mg of oral terbinafine to be effective in the treatment of tinea, but this effectiveness has not been compared with that of 250 mg.3,4 Moreover, doubling the dose increases the financial burden on patients and may also increase the likelihood of adverse effects.
Objectives
We aimed to compare the efficacy and safety of oral terbinafine 250 mg once daily versus 500 mg once daily in the treatment of tinea corporis and tinea cruris. We were unable to find any previous study that compared the clinical efficacy of these two regimens of oral terbinafine in the treatment of tinea corporis and tinea cruris.
Methods
Study design
The study was designed as an institution-based, assessor-blinded, parallel-group clinical trial with random treatment allocation in the dermatology outpatient department of the Medical College and Hospital, Kolkata between June 2018 and November 2018. Clearance from the institutional ethics committee was obtained prior to the commencement of the study.
Participants
Patients of either sex, between ages 18 and 60 years with tinea corporis, tinea cruris or both were recruited for the study. Consecutive patients who were treatment-naive and old cases who had been off any form of treatment for the previous one month (topical or oral) were recruited. Pregnant, lactating, immunocompromised patients, those with known liver disease or known hypersensitivity to terbinafine were not included. Patients with deranged baseline liver function tests were also excluded from the study.
At the screening visit, patients were enrolled based on inclusion criteria. Written informed consent was obtained. Detailed clinical data including the number of lesions, site/s of involvement and lesion activity were noted in the case record form. Number of lesions was categorised as single, few (2-4), moderate (4-6) and extensive (>6). Patients’ skin scraping samples were collected from at least two sites (if number of lesions >2). Skin scraping was collected on a black chart paper to facilitate easy visualisation against a dark background. The paper was folded tightly, and name of patient and outdoor registration number was written. The pooled sample was then sent for 10% KOH mount and culture on Sabouraud’s dextrose agar with chloramphenicol and cycloheximide. Baseline hemogram, serum urea and creatinine, and liver function tests were also done.
Interventions
Medicines were provided to the patients by the treating physician with either code medicine A (250 mg oral terbinafine daily) or medicine B (500 mg oral terbinafine daily) according to randomisation. Courses of medicines were provided till next visit. Patients in both arms were also prescribed topical clotrimazole cream 1% to be applied twice daily on affected areas and oral cetirizine 10 mg, one tablet at bedtime.
Outcome
Two follow-up visits were scheduled at two weeks’ intervals each. At every follow-up, patients were graded for improvement in signs and symptoms of each clinical parameter. Erythema, lesional papules/pustules/vesicles and scaling were visually graded as absent, mild, moderate and severe and compared at each visit to assess improvement. The overall improvement was graded on an ordinal with five categories:
Grade I (worsening), grade II (no improvement), grade III (25% improvement), grade IV (50% improvement), grade V (75% improvement) and grade VI (100% improvement). Intensity of itch was indicated on a visual analogue scale by the patient. The highest grade for clinical improvement was given only when lesion was 100% cured (clinically + symptomatically + mycologically). The assessment was done by an assessor who was blinded to the treatment received by the patient. Two assessors assessed every patient for clinical improvement throughout the study period.
A KOH mount was done on every follow-up to assess mycological cure. Sample was collected by two investigators. Blade no. 22 was used. Microscopy was done by the microbiologist in the department of microbiology who was also blinded to the treatment received by the patient. Fungal culture was repeated at the end of four weeks. Repeat blood tests were done at the end of four weeks to assess for any side effects.
Response to treatment was assessed at the end of four weeks and was categorised into no resolution (no clinical improvement and KOH mount/ culture positive), partial resolution (partial clinical improvement ± KOH mount positive ± culture positive), complete resolution (complete clinical cure, plus both KOH mount and culture negative). Comparison of liver function tests at baseline and at the end of treatment was done to evaluate the safety 250 mg and 500 mg of oral terbinafine.
Sample size
Since we intended this as a pilot study, sample size calculation statistics was not performed. We recruited 30 patients in each intervention arm.
Randomisation
Sequence generation
Eligible patients were randomised into either group A (250 mg oral terbinafine daily) or group B (500 mg oral terbinafine daily) with an allocation ratio 1:1, as per a computer-generated random number table generated by an independent co-ordinator.
Allocation concealment
This was done by the sequentially numbered opaque sealed envelope (SNOSE) technique.
Implementation
After thorough evaluation, the patient was referred to IC who assigned the treatment using SNOSE technique. The treating physician then dispensed the “medication A (250mg)” or “medication B (500mg)” and then outcome and adverse effects were assessed byban assessor.
Blinding
The assessor and the individual who performed the statistical analysis were both blinded to the treatment arms and identified the treatment received by the patients in terms of code.
Statistical methods
Continuous efficacy variables were compared between groups by independent samples t-test and within group by paired t-test. Mann-Whitney U test and Wilcoxon’s test were carried out for unpaired and paired non-parametric data. Categorical data were compared between groups by chi-squared test or Fisher’s exact test, as appropriate. Freidman’s ANOVA was carried out with non-parametric data for within-group repeated measures comparisons. Data was entered in Microsoft Excel and analysis was done with the help of statistical software Medcalc® v 17.0.0.
All the data were processed and analysed at the Department of Dermatology, and analysis was made only after the case record form of the last subject was in, data had been entered, and the computer database cleaned and locked. For analysis of efficacy of treatment, only those patients who had completed all follow up visits were included. But for analysis of adverse effects of the drug, we included patients who had taken at least one dose of treatment.
Results
Participant flow
The flow of study participants is shown in Figure 1. Two participants from group B were lost after the first follow-up, and three participants from group A and another four participants from group B were lost after the second follow-up. Hence at the end of the study, the results for 27 participants from group A and 24 participants from group B were analysed. However, following the modified intention-to-treat strategy, the last observation of lost patients was carried forward and all 60 participants were analysed.
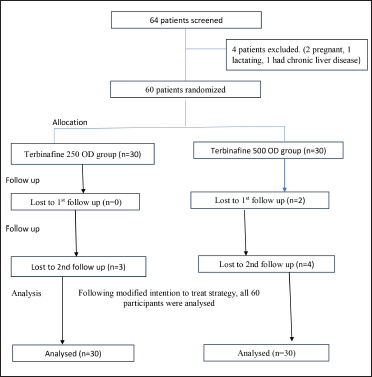
- Flowchart of study participants
Recruitment
Sixty patients were randomised equally into two groups. Females outnumbered males, but the difference between the groups was not significant (P = 0.128). Both groups were comparable with respect to demographic parameters like age, residence (rural/ urban), literacy, occupation and family history of tinea [Table 1].
| Parameters | Terbinafine 250 mg daily (n = 30) |
Terbinafine 500 mg daily (n = 30) |
P-value (between groups) |
|---|---|---|---|
|
Age (years) Mean ± SD Median |
35.43 ± 12.7 34 |
36.23 ± 13.1 36 |
0.81 |
|
Gender Male Female |
11 (36.7%) 19 (63.3%) |
15 (50%) 15 (50%) |
0.13 |
|
Residence Rural Urban |
14 (46.7%) 16 (53.3%) |
15 (50%) 15 (50%) |
1.00 |
|
Occupation Housewife Retired Small-skilled Business Teacher |
18 (60%) 1 (3.3%) 5 (16.7%) 1 (3.3%) 5 (16.7%) |
13 (43.3%) 0 7 (23.3%) 1 (3.3%) 9 (3%) |
0.62 |
**SD = Standard deviation; P value was obtained from Student’s unpaired t-test for age; Fisher’s exact test for gender distribution, residence and family history
Baseline data
Baseline clinical parameters such as disease duration, lesional activity (papules, vesicles, pustules), degree of erythema and scaling, severity of itching, type of tinea (tinea corporis/ tinea cruris/ both), and presence/absence of co-morbidities were comparable in both treatment arms [Table 2]. There was no statistically significant difference in culture result (growth/no growth) and species grown between the two groups [Table 2].
| Parameters | Terbinafine 250 mg daily (n = 30) | Terbinafine 500 mg daily (n = 30) |
P-value (Between groups) |
|---|---|---|---|
| Duration in months | 4.58 ± 4.74 | 4 ± 3.3 | 0.75 |
|
Clinical diagnosis Tinea corporis Tinea cruris Tinea corporis + Tinea cruris |
5 (16.7%) 0 25 (83.3%) |
5 (16.7%) 1 (3.3%) 24 (80%) |
0.60 |
|
Number of lesions Single Few Significant Extensive |
1 (3.3%) 4 (13.3%) 22 (73.3%) 3 (10%) |
0 11 (36.7%) 16 (53.3%) 3 (10.0%) |
0.16 |
|
Itching (visual analog scale) Median |
8.5 | 8 | 0.05 |
|
Erythema None Mild Moderate Severe |
1 (3.3%) 5 (16.7%) 10 (33.3%) 14 (46.7%) |
0 5 (16.7%) 13 (43.3%) 12 (40%) |
0.62 |
|
Scaling Mild Moderate Severe |
3 (10%) 14 (46.7%) 13 (43.3%) |
6 (20%) 10 (33.3%) 14 (46.7%) |
0.43 |
|
Culture Negative Positive |
4 (13.3%) 26 (86.7%) |
4 (13.3%) 26 (86.7%) |
1.00 |
|
Species No growth Trichophyton rubrum Trichophyton mentagrophytes Trichophyton tonsurans |
4 (13.3%) 3 (10%) 17 (56.7%) 6 (20%) |
4 (13.3%) 5 (16.7%) 18 (60%) 3 (10%) |
0.67 |
| Co-morbidities Present Absent |
6 (20%)* 24 (80%) |
7 (23.3%)# 23 (76.7%) |
1.00 |
*Diabetes mellitus = 2, Hypertension = 1, Hypothyroidism = 2, Bronchial asthma = 1; #Diabetes mellitus = 4, Hypertension = 1, Bronchial asthma = 1, Hypothyroidism+ Hypertension = 1, P value was obtained from Mann-Whitney U test for duration and visual analog scale score, chi-square test for diagnosis, co-morbidities, type of lesions, erythema, scaling, fungal culture and species identification
Outcomes and estimation
At the end of two weeks of treatment (first follow-up), clinical parameters were compared and no statistically significant differences were found in reductions in erythema (P = 0.29), scaling (P = 0.29) and lesion activity (papules, vesicles, pustules; P = 0.34) between the two groups [Table 3]. However, itching reduced significantly in the group receiving 250 mg terbinafine daily (P = 0.04). There was no difference in mycological clearance on the KOH mount at the end of two weeks (13.8% (250 mg daily group) and 14.3% (500 mg daily group); P = 1.000)).
| Parameters | First follow-up (after 2 weeks of treatment) | Second follow-up (after 4 weeks of treatment) | ||||
|---|---|---|---|---|---|---|
| Terbinafine 250 mg daily n = 29 | Terbinafine 500 mg daily n = 28 | P value | Terbinafine 250 mg daily n = 27 | Terbinafine 500 mg daily n = 24 |
P-value | |
|
Itching (visual analog scale score) Median |
5 |
6 |
0.98 (per protocol & intention to treat) |
6 |
6 |
0.69 (per protocol) 0.87 (per intention to treat) |
|
Itching Grade I (Worsened) Grade II (No improvement) Grade III (25% improvement) Grade IV (50% improvement) Grade V (75% improvement) Grade VI (100% improve-ment) |
5 (17.2%) 5 (17.2%) 6 (20.7%) 1 (3.4%) 10 (34.5%) 2 (6.9%) |
3 (10.7%) 8 (28.6%) 7 (25%) 4 (14.9%) 1 (3.6%) 5 (17.8%) |
0.04 (per protocol) 0.034 (per intention to treat) |
8 (29.6%) 9 (33.3%) 5 (18.5%) 1 (3.7%) 0 4 (14.8%) |
6 (25%) 6 (25%) 3 (12.5% 0 2 (8.3%) 7 (29.1%) |
0.41 (per protocol) 0.95 (per intention to treat) |
|
Erythema Grade I (Worsened) Grade II (No improvement) Grade III (25% improvement) Grade IV (50% improvement) Grade V (75% improvement) Grade VI (100% improve-ment) |
3 (10.3%) 4 (13.8%) 5 (17.2%) 6 (20.7%) 8 (27.6%) 3 (10.3%) |
0 2 (7.1%) 3 (10.8%) 5 (17.9%) 1 0 (35.7%) 8 (28.6%) |
0.24 (per protocol) 0.29 (per intention to treat) |
1 (3.7%) 10 (37.03%) 7 (25.4%) 2 (7.4%) 1 (3.7%) 6 (22.2%) |
0 9 (37.5%) 2 (8.3%) 2 (8.3%) 2 (8.3%) 9 (37.5%) |
0.47 (per protocol) 0.41 (per intention to treat) |
|
Scaling Grade I (Worsened) Grade II (No improvement) Grade III (25% improvement) Grade IV (50% improvement) Grade V (75% improvement) Grade VI (100% improve-ment) |
2 (6.9%) 0 5 (17.2%) 7 (24.1%) 10 (34.5%) 5 (17.2%) |
0 3 (10.7%) 7 (25%) 8 (28.6%) 6 (21.5%) 4 (14.3%) |
0.26 (per protocol) 0.29 (intention to treat) |
4 (14.8%) 11 (40.8%) 2 (7.4%) 3 (11.1%) 1 (3.7%) 6 (22.2%) |
3 (12.5%) 4 (16.6%) 5 (20.8%) 2 (8.3%) 0 10 (41.7%) |
0.24 (per protocol) 0.45 (per intention to treat) |
|
Lesions improvement Grade I (Worsened) Grade II (No improvement) Grade III (25% improvement) Grade IV (50% improvement) Grade V (75% improvement) Grade VI (100% improve-ment |
2 (6.9%) 3 (10.3%) 5 (17.2%) 8 (27.6%) 7 (24.1%) 4 (13.8%) |
0 2 (7.1%) 7 (25%) 4 (14.3%) 6 (21.5%) 9 (32.1%) |
0.32 (per protocol) 0.34 (per intention to treat) |
5 (18.5%) 7 (25.4%) 6 (22.2%) 3 (11.1%) 0 6 (22.2%) |
3 (12.5%) 5 (20.8%) 2 (8.3%) 6 (25%) 1 (4.2%) 7 (29.2%) |
0.45 (per protocol) 0.52 (per intention to treat) |
|
KOH mount No hyphae Hyphae seen |
4 (13.8%) 25 (86.2%) |
4 (14.3%) 24 (85.7%) |
1.00 |
7 (25.9%) 20 (74.1%) |
8 (33.3%) 16 (66.7%) |
0.76 |
|
Culture No growth Growth present |
Not done at first follow-up |
9 (33.3%) 18 (66.7%) |
9 (37.5%) 15 (62.5%) |
P = 0.78 |
||
P value was obtained from Mann-Whitney U test for visual analog scale score (chi-square test for itching, erythema, lesions and scaling improvement, Fisher’s exact test for detection of fungal element in KOH mount, and adverse drug reaction
Similarly, at the end of four weeks (second follow-up) no significant differences in improvement of clinical or mycological parameters were seen between the two treatment arms. There was no further improvement in itching (P = 0.95), nor were there statistically significant differences in improvement in erythema (P = 0.41), scaling (P = 0.45) and lesion activity (papules, vesicles, pustules, P = 0.52) between the two treatment arms [Table 3].
No significant difference in mycological cure was seen between the two groups. KOH negativity was reported in only 25.9% (250 mg daily group) and 33.3% (500 mg daily group) after four weeks of treatment (P = 0.76). Culture negativity was seen in 33.3% (250 mg daily group) and 37.5% (500 mg daily group), a difference which was not statistically significant (P = 0.78). Overall cure rates were 20% and 33.3% in the two treatment arms respectively; the higher dose of terbinafine did not provide a statistically significant better cure than the lower dose (P = 0.82) [Tables 4 and 5].
| Disease activity at the end of 4 weeks | Terbinafine 250 mg daily (n = 27)* |
Terbinafine 500 mg daily (n = 24)* |
P = 0.19 (chi-square test) |
|---|---|---|---|
| No resolution | 3 (11.1%) | 0 | |
| Partial resolution | 18 (66.7%) | 16 (66.7%) | |
| Complete resolution | 6 (22.2%) | 8 (33.3%) |
| Disease activity at the end of 4 weeks | Terbinafine 250 mg daily (n = 30)* |
Terbinafine 500 mg daily (n = 30)* |
P = 0.82 (chi-square test) |
|---|---|---|---|
| No resolution | 6 (20%) | 6 (20%) | |
| Partial resolution | 18 (60%) | 16 (66.7%) | |
| Complete resolution | 6 (20%) | 8 (33.3%) |
Ancillary analysis
Intragroup analysis for group A (250 mg oral terbinafine daily)
There were significant improvements in itching (P = 0.0028) and scaling (P = 0.001) between the first and second follow-ups. However, improvements in erythema and lesional activity were not significant. Improvement in the mycological cure rate between the first and second follow-ups was statistically significant (P = 0.01 and 0.03 for KOH and culture negativity, respectively). Of the three main species isolated (Trichophyton rubrum, Trichophyton mentagrophytes, Trichophyton tonsurans), the maximum response to treatment was seen in infections with Trichophyton mentagrophytes (P = 0.01).
Intragroup analysis for group B (500 mg oral terbinafine daily)
Contrary to the findings in group A, there was no significant improvement between the first and second follow-ups in itching (P = 0.79) and scaling (0.72). There was no significant improvement in lesion activity either (P = 0.06). Significant improvement in erythema was however seen (P = 0.04). Increase in mycological cure was also significant between the first and second follow-ups (P for KOH negativity = 0.005, P for culture negativity = 0.03). As in group A, the maximum response to therapy was seen where Trichophyton mentagrophytes was isolated (P = 0.001).
Adverse effects
Adverse effects were reported by three patients. Two patients who received 250 mg of the drug complained of nausea and one patient who received 500 mg complained of loss of appetite. After completion of the study, repeat liver function tests were done and found to be deranged in two patients who had received 250 mg terbinafine daily (alkaline phosphatase was raised, 170 and 184 IU/L, respectively). The difference with respect to adverse reaction incidence between the two treatment arms was not statistically significant (P = 1.00)
Discussion
There has been a drastic change in the Indian scenario of dermatophytosis, with increase in its occurrence, relapse, recurrence and chronicity in recent years. Terbinafine is one of the most commonly used antifungal drugs in the treatment of superficial fungal infections because of its broad-spectrum fungicidal activity.3 However, recently clinical failure and relapses have been observed with terbinafine therapy of dermatophytosis. Of late, dermatologists are sometimes upscaling the dosage of terbinafine to 500 mg daily. We conducted this trial to evaluate whether or not a higher dose of the drug has a significant benefit over the conventional dose.
Among the 60 participants enrolled in our study, females marginally outnumbered males, though earlier studies reported a predominance of males.4 In a recent report Verma et al. have postulated that changing fashion trends in women like body-hugging jeans, jeggings and leggings lead to more sweating and occlusion.5
The most common clinical variant was combined tinea corporis and cruris (81.7%) which is in accordance with previous reports by Babu et al. and Singh et al.3,4 Fungal growth on culture in our study was seen in 86.7% of patients; previous studies have reported yields of 73.68% (Singh et al.),6 76.38% (Bindu et al.),7 and 85.9% (Poluri et al.).6 Trichophyton mentagrophytes was the most common species isolated in our study. Previously Trichophyton rubrum used to be the most common species but some recent studies found Trichophyton mentagrophytes dominant.6,8 However Das et al. have reported Trichophyton verrucosum as the most common species in their study.8
Studies have reported cure rates of only 37%,9 of 30%4 and 23%4 with oral terbinafine 250 mg daily at the end of four weeks. In our study the cure rate was even lower, at 20% at this dose.
The cure rate with oral terbinafine 500 mg daily at the end of four weeks was 33.3% in our study. This is similar to the findings by Singh et al. who reported a cure rate of 33% with terbinafine 500 mg daily.4 However Bhatia et al. have reported a cure rate of 74%.9 In a retrospective study by Babu et al, 80% of patients showed more than 75% improvement with terbinafine 500 mg daily.3 The wide variation in cure rate could be possible because of following reasons: (1) In our study highest grading for clinical cure was given only when there was 100% resolution of lesion clinically in addition to when both KOH and culture negativity was achieved. In the study by Babu et al., clinical resolution of >75% in lesions was given the highest grading. (2) Study by Babu et al. was a questionnaire-based survey conducted among doctors; hence bias cannot be ruled out. (3) Other studies have not taken into consideration the use of topical antifungals. Whether topical antifungal/s was/were used or not, or same topical antifungal was used is not known, thus affecting the study outcome.
The sudden increase in the prevalence of recalcitrant dermatophytosis is a matter of concern. Antifungal resistance in dermatophytes is also emerging rapidly, especially to allylamines. Trichophyton mentagrophytes which has emerged as the dominant pathogen has been found to exhibit a rapid growth in primary culture within 5-7 days.8 Terbinafine resistance in Trichophyton mentagrophytes complex and Trichophyton rubrum has been ascribed to a point mutation in the squalene epoxidase gene, the gene essential for the ergosterol biosynthesis.10
Babu et al. have reported daily single dose of 500 mg terbinafine to be efficacious in 92% of patients with dermatophytosis. Since no comparison with the conventional dose was done, it is difficult to say if doubling the dose had any added benefit in their subjects. Previous studies have shown that terbinafine, even at conventional doses, achieves skin levels that exceed trough plasma levels by a factor of 75, well above the minimum inhibitory concentration for dermatophytes. A higher dose, if warranted, should perhaps be in divided doses (250 mg twice daily) rather than a single dose of 500 mg.11
Limitations
Participants were not followed up to see if a particular dose of terbinafine provided longer remissions than the other. Also, clinical assessments were subjective which could have possibly affected the study outcome.
Conclusion
Up-dosing of oral terbinafine to 500 mg daily did not have any additional benefit over 250 mg taken daily and neither dosage in combination with topical clotrimazole provided a satisfactory cure rate in the treatment of dermatophytosis.
Trial registration: CTRI/2018/07/015131
Acknowledgement
We are grateful to Messrs Glenmark Pharmaceuticals Ltd for kindly providing the medicines for the study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
The authors declare no conflict of interest.
References
- Superficial fungal infections: Dermatophytosis, Onychomycosis, Tinea Nigra, Piedra In: Wolff K, Goldsmith LA, Katz SI, Gilchrist BA, Paller AS, Lefell DJ, eds. Fitzpatrick’s Dermatology in General Medicine (7th ed.). McGraw-Hill; 2008. p. :1807-14.
- [Google Scholar]
- The menace of chronic and recurrent dermatophytosis in India: Is the problem deeper than we perceive? Indian Dermatol Online J. 2016;7:73-6.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of terbinafine 500 mg once daily in patients with dermatophytosis. Indian J Dermatol. 2017;62:395-9.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of terbinafine and itraconazole in different doses and in combination in the treatment of tinea infection: A randomized controlled parallel group open labelled trial with clinico-mycological correlation. Indian J Dermatol. 2020;65:284-9.
- [CrossRef] [PubMed] [Google Scholar]
- The great Indian epidemic of superficial dermatophytosis: An appraisal. Indian J Dermatol. 2017;62:227-36.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinicomycological study of dermatophytosis in a tertiary care hospital in eastern India: A cross-sectional study. Indian Dermatol Online J. 2020;11:46-50.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-mycological study of dermatophytosis in Calicut. Indian J Dermatol Venereol Leprol. 2002;68:259-61.
- [PubMed] [Google Scholar]
- The current Indian epidemic of dermatophytosis: A study on causative agents and sensitivity patterns. Indian J Dermatol. 2020;65:118-22.
- [CrossRef] [Google Scholar]
- Efficacy of oral terbinafine versus itraconazole in treatment of dermatophytic infection of skin - A prospective, randomized comparative study. Indian J Pharmacol. 2019;51:116-9.
- [CrossRef] [PubMed] [Google Scholar]
- Mutation in the Squalene Epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with allylamine resistance. Antimicrob Agents Chemother. 2018;62:e02522-17.
- [CrossRef] [PubMed] [Google Scholar]
- Rational for drug dosimetry and duration of terbinafine in the context of recalcitrant dermatophytosis: Is 500 mg better than 250 mg OD or BD? Indian J Dermatol. 2017;62:665-7.
- [CrossRef] [PubMed] [Google Scholar]






