Translate this page into:
Update for dermatologists on monkeypox: An emerging health problem in the world
Corresponding author: Dr. Keshavamurthy Vinay, Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. vinay.keshavmurthy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mustari AP, Bishnoi A, Khanna U, Vinay K. Update for dermatologists on monkeypox: An emerging health problem in the world. Indian J Dermatol Venereol Leprol 2023;89:326-31.
Introduction
As the incidence of severe coronavirus disease 2019 is declining in most countries, the world is anticipating another pandemic reflected by increasing cases of monkeypox globally.1 Monkeypox is historically a zoonotic viral disease caused by Orthopoxvirus and is endemic in central and west Africa.2 It is clinically characterised by prodromal symptoms including fever, lymphadenopathy, enanthem and exanthem.3 The first human case was confirmed in 1970 in Bokenda, a remote village in the Democratic Republic of Congo, in a child suspected of having smallpox.4 Monkeypox and smallpox are closely related viruses and immunity against smallpox confers immunity to monkeypox. Smallpox eradication and cessation of its vaccination have probably heralded the outbreaks of monkeypox globally, including in India.
The World Health Organization declared the current global monkeypox outbreak as a public health emergency of international concern on July 23, 2022.5 As of July 26, 2022, more than 19,000 cases have been reported globally.6 The first case of monkeypox in India was confirmed in Kerala on July 13, 2022, and subsequently, 11 more cases were reported in the following weeks.
In this focused review on monkeypox, we highlight the causative agent, clinical features, differential diagnoses, treatment options, complications and control and prevention strategies for emerging monkeypox disease. We also discuss the logistic issues specific to India, such as sample collection.
Causative agent and transmission
Monkeypox virus is a double-stranded DNA virus of Poxviridae family, Chordopoxvirinae subfamily and Orthopoxvirus genus.7 This virus has two clades, West African and Congo Basin. The majority of cases in this ongoing epidemic are due to the West African clade, which is less virulent compared to the Congo Basin clade. The natural reservoirs are African dormice, rope and sun squirrels and the virus is transmitted through respiratory droplets, fomites, body fluids and contact or inhalation of aerosols or vesicular fluid. Transmission can also occur through bite, scratch or close contact with infected animals such as monkeys, rats, apes and squirrels or consumption of their raw meat. The risk of exposure to monkeypox virus is stratified into higher, intermediate, lower and no risk [Table 1].8 The secondary attack rate is around 10%. The mortality rate in the previous outbreaks in endemic areas was estimated to be around 1-11% in unvaccinated patients.9
| Risk category | Degree of exposure | Management | |
|---|---|---|---|
| Monitoring$ | PEP¶ | ||
| Higher | Exposure to broken skin or mucus membrane with skin lesions or body fluids from a patient with monkeypox OR Any sexual or close contact involving mucus membranes# with a patient with monkeypox OR Exposure to broken skin or mucus membrane with contaminated materials* |
Yes | Yes |
| Intermediate | Unmasked exposure (within 6 feet for ≥3 hours) to a patient with monkeypox virus OR Exposure to intact skin with skin lesions or body fluids from a patient with monkeypox OR Exposure to intact skin with contaminated materials* OR Exposure to individual’s clothing with skin lesions, body fluids or contaminated materials* from a patient with monkeypox |
Yes | BOR |
| Lower | Exposure to the living space of a patient with monkeypox in the absence of ‘higher’ and ‘intermediate’ risk exposure | Yes | No |
| No risk | No exposure to skin lesions or body fluids, contaminated materials* and living space | No | No |
*Contaminated materials include linen, clothing or sex toys, #Kissing, oro-anal, oro-genital, vaginal or peno-anal, $Watch for signs and symptoms for 21 days from the last exposure, ¶PEP, post-exposure prophylaxis with smallpox vaccines (JYNNEOS or ACAM2000), BOR, Benefits outweigh the risk
Risk factors
Risk factors for infection in previous outbreaks have been male sex, age >18 years, malnutrition, handling of sick animals, immunocompromised status and unvaccinated status for smallpox.10 The protective efficacy of the smallpox vaccine or natural infection against monkeypox is around 85%.11
Clinical features
After a variable incubation period of 5-21 days, a patient presents with fever associated with chills, diaphoresis, headache, sore throat, cough, malaise and characteristic lymphadenopathy. After 1-3 days of fever, the patient develops multiple painful monomorphic deep umbilicated vesico-pustules sized 1-2 centimetres with white halo and surrounding erythema over face, palm, soles and mucous membranes (oral, genital and ocular). The lesions evolve through various stages including macules, papules, vesicles and pustules, each lasting for 1-2 days [Figure 1]. These lesions coalesce to form larger firm dry crusted plaques which resolve spontaneously in 3-4 weeks with scarring. Inoculation via invasive routes is associated with severe disease and shorter incubation period. The patient ceases to be infectious once the lesions crust off.3,12
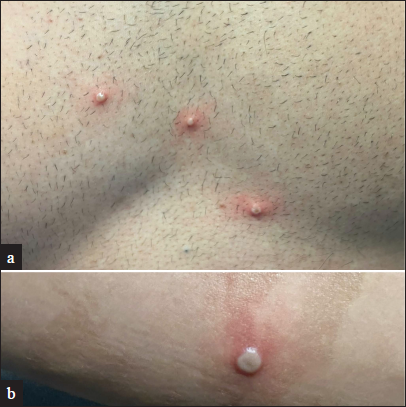
- Cutaneous examination in a 30-year-old HIV-positive individual with undetectable viral load. Discrete vesiculo-pustules with an umbilicated centre and a prominent erythematous rim are seen on the chest (a) and right forearm (b). Polymerase chain reaction (PCR) testing from a lesional swab detected non-variola orthopox virus DNA
The current outbreak differs from prior monkeypox outbreaks which mainly involved zoonotic infections. Previously, monkeypox in humans was reported either in travellers returning from Africa (two cases in the US in 2021) or due to animal-human transmission through imported animals from endemic regions (outbreak in the US Midwest in 2003).13 The current outbreaks are chiefly being reported in non-endemic areas without any travel history.14 Human-to-human transmission during the 2022 outbreak is occurring due to intimate skin contact during sexual activities. The majority of cases are among men having sex with men with high-risk behaviour (multiple sexual partners and drug abuse), are co-occurring with other sexually transmitted infections and genital involvement is a common feature. The west African clade is responsible for a large proportion of cases in the current outbreak, thus patients have mild systemic symptoms and the mortality rate is low. Homogenous papules and pseudopustules are the commonest features and macular lesions and mucosal ulcers (pharyngeal ulcers and proctitis) are infrequent in the current outbreak. Similar to other poxviruses, localisation of lesions at the site of inoculation is common. Smallpox vaccine and human deficiency virus infection are not associated with a marker of severity. Pain, dysphagia due to pharyngeal oedema and ocular involvement (conjunctivitis) are common reasons for hospitalisation in the current outbreak.15 Case definition for monkeypox outbreak in India is summarised in Table 2.16
| Suspected case |
| Individuals with a travel history to affected countries in the last 21 days presenting with unexplained rash and ≥1 following signs or symptoms: swollen lymph nodes, fever, headache, body aches or profound weakness |
| Probable case |
| Individuals with clinical features of monkeypox and epidemiological link (face-face exposure, direct physical contact, contact with contaminated objects such as cloths or utensils) |
| Confirmed case |
| Case with demonstrated monkeypox DNA by PCR or sequencing* |
PCR: polymerase chain reaction, *In the absence of recent Vaccinia vaccination
Complications
Complications include secondary infection, hyper or hypopigmentation, scarring, keratitis, corneal scarring, dehydration, encephalitis, bronchopneumonia, myocarditis, acute kidney injury, epiglottitis, sepsis and death.
Differential diagnoses
The common differential diagnoses include smallpox, chickenpox (varicella), disseminated zoster, disseminated herpes simplex, syphilis, yaws, measles with secondary cutaneous infection, infectious mononucleosis, molluscum contagiosum, hand-foot-mouth disease and deep fungal infections (histoplasmosis, cryptococcosis and penicilliosis). The differentiating features of monkeypox from its close mimickers are summarised in Table 3.17-21
| Disease | Causative organism: genus; species | Incubation period | Age group and route of transmission | Symptoms and distribution | Morphology |
|---|---|---|---|---|---|
| Monkeypox | Orthopoxvirus; Monkeypox virus | 5-21 days | Variable Respiratory droplets, fomites, direct contact with body fluids and contact or inhalation of aerosols or from vesicular fluid with SAR of around 10% | Pain in the active phase Itching in the recovery phase Centrifugal (predominantly face and distal extremities) |
Monomorphic deep umbilicated vesico-pustules of size 1-2 centimetres with white halo and surrounding erythema over face, palm and soles and mucus membranes [Figure 1] |
| Chickenpox | Varicella-zoster virus Human herpesvirus 3 |
10-21 days | Children and adults Direct contact and respiratory droplets with SAR 60-100% |
Itching Centripetal |
Multiple vesicles of size 2-4 millimetres on an erythematous base containing clear fluid at different stages of evolution (polymorphic) |
| Disseminated herpes zoster | Varicella-zoster virus Human herpesvirus 3 |
Reactivation of HHV 3 | Elderly Reactivation of HHV 3 | Burning Cervical and thoracic | Multiple round umbilicated grouped vesicles on an erythematous base of varying size (differentiate from herpes simplex) with more than 20 extradermatomal vesicles |
| Disseminated herpes simplex | SimplexvirusHuman herpesvirus 1 | 2-12 days | Neonates Direct contact, fomites and transplacental in neonates |
Painful Generalised |
Multiple monomorphic vesicles of uniform size (differentiate from herpes zoster) |
| Measles |
Morbillivirus Measles morbillivirus |
7-21 days | Young children Respiratory droplets and direct contact with SAR >90% |
Nonpruritic Cephalocaudal (characteristic) | Erythematous maculopapular blanchable rash with enanthem (Koplik spots) |
| Hand-foot-mouth disease | Picornaviridae Coxsackievirus A16 and Enterovirus Enterovirus A71 |
3-6 days | Young children (<5 years) Feco-oral, fomites and droplets |
Pruritic Acral areas: hands, feet, oral cavity and buttocks |
Multiple elliptical vesicles (long axis along skin lines) of size 2-4 millimetres with surrounding erythema and nail changes (leukonychia and Beau lines to partial or complete onychomadesis) |
| Secondary syphilis | Treponema Treponema pallidum |
9-90 days | Adults Sexual route, direct contact, blood transmission, transplacental and rarely needle-stick injury | Asymptomatic Oral cavity, genitals, palm and soles | Multiple pink to white moist flat-topped papules and plaques over genitals (condyloma lata) Mucous patches, snail track ulcers and split papules Psoriasiform annular coppery-brown plaques over palm and soles |
| Molluscum contagiosum | Molluscipoxvirus Molluscum contagiosum virus |
6 days-6 months | Children, young adults and immunocompromised individuals Direct contact and sexual route |
Asymptomatic > pruritic Any site (usually face and genitals) |
Multiple dome-shaped to umbilicated, pearly white to pink waxy papules and plaques of size 2-5 millimetres |
| Yaws | Treponema Treponema pallidum pertenue |
9-90 days | <15 years (peak in 6-10 years) Direct skin-to-skin contact |
Usually asymptomatic Lower extremities |
Solitary nodules or multiple ulcerative yellowish papules of size 2-5 centimetres with characteristic granulation tissue at the base with elevated borders |
| Infectious mononucleosis |
Lymphocryptovirus Human gammaherpesvirus 4 |
4-6 weeks | 15-24 years Direct contact and body fluids especially saliva |
Nonpruritic Trunk and upper extremities | Widespread scattered maculopapular rash over trunk and extremities with palatal enanthem and cervical lymphadenopathy |
| Histoplasmosis | Histoplasma Histoplasma capsulatum |
7-21 days | Adults (3rd decade) Inhalation of fungal spores commonly found in bird and bat droppings |
Asymptomatic Face, trunk, extremities and oral mucosa | Umbilicated and crusted papules, plaques, nodules, ulcers, rosacea-like, acneiform, and molluscum-like lesions of size 0.5-1.5 centimetres in an immunocompromised patient |
| Cryptococcosis | Cryptococcus Cryptococcus neoformans |
2-12 months | Adults (3rd-5th decade) Inhalation of fungal spores commonly found in bird and bat droppings and unwashed raw fruit |
Asymptomatic Face, extremities, trunk and oral mucosa | Umbilicated crusted papules, plaques, acneiform papules, pustules, draining sinuses, warty vegetating plaques, nodules and ulcers in an immunocompromised patient |
| Penicilliosis | Talaromyces Talaromyces marneffei |
6-12 months | Adults (3rd-5th decade) Inhalation of spores from the soil |
Asymptomatic Face, neck, and trunk | Umbilicated flesh-coloured necrotic papules and ulcers in an immunocompromised patient |
VZV: Varicella-zoster virus, SAR: Secondary attack rate, HHV 3: Human herpesvirus 3
Laboratory diagnosis
Nucleic acid detection tests are the gold standard for the diagnosis of monkeypox. Serology can be used in the convalescent phase. Health care workers should adopt adequate precautions while collecting samples. Recommended personal protective measures while collecting samples include gown, face shield or safety goggles, N-95 mask and double gloves. The samples needed in a suspected case of monkeypox are summarised in Table 4.16
| Active stage | Recovery stage | |
|---|---|---|
| Symptomatic## |
#Lesion roof with a scalpel or plastic scrapper in a plain tube #Lesion roof with a scalpel or plastic scrapper in a plain tube #Vesicular fluid with an intradermal syringe #Vesicular fluid with an intradermal syringe #Scrapings from the base of the lesions with sterile polyester swabs collected in a plain tube #Scrapings from the base of the lesions with sterile polyester swabs collected in a plain tube #Crust in a plain tube #Crust in a plain tube NPS/OPS in a plain tube Blood in SSGT (4-5 mL) and EDTA (2-3 mL) vials Blood in SSGT (4-5 mL Blood in SSGT (4-5 mL) and EDTA (2-3 Blood in SSGT (4-5 mL) and EDTA (2-3 mL Blood in SSGT (4-5 mL) and EDTA (2-3 mL) vials Urine in a sterile urine container (3-5 mL) Urine in a sterile urine container (3-5 mL Urine in a sterile urine container (3-5 mL) |
Blood in SSGT (4-5 mL) Urine in a sterile urine container (3-5 mL) Urine in a sterile urine container (3-5 mL Urine in a sterile urine container (3-5 mL) |
| Asymptomatic | Watch for signs and symptoms for 21 days from the last exposure If any clinical features, collect the sample as mentioned above |
|
EDTA: Ethylenediamine tetra acetic acid, SSGT: Serum separation gel tube, OPS: Oropharyngeal swabs, NPS: Nasopharyngeal swabs, #The samples should be collected from multiple sites, ##Clinical images should be mailed to hellopragya22@gmail.com or dr.rima.sahay@gmail.com, *Clinical samples should be sent to Dr. Pragya D. Yadav, Scientist ‘F’ & Group Leader, Maximum Containment Facility, ICMR-NIV, Microbial Containment Complex, 130/1, Sus Road, Pashan, Pune 411021. Tel: Office: 020-26006111 OR Dr. Rima R. Sahay, Scientist ‘C’, Maximum Containment Facility, ICMR-NIV, Microbial Containment Complex, 130/1, Sus Road, Pashan, Pune 411021. Tel: Office: 020-26006160
Whom to test: Samples should be collected in only symptomatic patients, suspected of having monkeypox infection.16
Samples from the lesions: The lesional samples are collected in plain vials from multiple sites. After cleaning the lesions with 80% alcohol, the vesicle is de-roofed using a sterile scalpel. Fluid from vesicles or pustules is collected using 1 mL tuberculin syringe. Scraping from the lesions and crust is collected by gentle scraping with a sterile polystyrene swab [Table 4].16
Oropharyngeal swab (OPS) and nasopharyngeal swab (NPS): OPS and NPS are collected using a sterile polystyrene swab in a plain tube. OPS is collected from tonsillar pillars and posterior pharynx without touching buccal mucosa, tongue, gums or teeth. If there are oral lesions, samples should be collected from them. NPS is collected using a polystyrene swab with a long flexible shaft inserting into the nostril along the palate until resistance is met at turbinates.
Blood samples: Blood specimens are collected in ethylenediamine tetraacetic acid (2 mL; purple head) and serum separation gel tube (3 mL; yellow head) vial.
Urine sample: 3-5 mL of mid-stream urine is collected in a sterile urine container.
The samples should be transported at 4°C to National Institute of Virology (NIV), Pune, Maharashtra for further processing [Table 4]. The clinical samples will be subjected to a polymerase chain reaction (PCR) for orthopox viruses. If positive, confirmation will be done by monkeypox-specific PCR or monkeypox real-time PCR. In addition, viral isolation and next-generation sequencing of positive samples (MiniSeq and NextSeq) will be done for further classification. The turnaround time is around 48-72 hours.16
Prevention
Patient care
Patients not needing medical attention can be isolated at home. Patients should be isolated in a separate room with separate ventilation. All lesions should be covered with a sheet or gown and the patient should avoid contact with family members.
Risk of transmission in the healthcare setting
Monkeypox virus is transmitted by close skin-to-skin contact, physical contact with body fluids including saliva and semen and via respiratory secretion through face-face interaction. Health care workers should wear personal protective equipment which includes coveralls, N-95 masks, face shield or safety goggles and double pair of gloves. To prevent the risk of transmission, all skin lesions should be covered using long sleeves and long pants and a surgical mask (triple layer) should be worn at all times. Patients should be advised to avoid scratching or touching the lesions.16
Contact tracing
Contact tracing should be done to identify all possible contacts across households, workplaces, social gatherings or any other interactions. A contact is defined as an individual who had any of the following types of exposure to the index case from the day of the first symptom till all scabs have fallen off: face-to-face contact, close physical contact including sexual contact, or exposure to contaminated materials. Asymptomatic contacts should be observed every day for the development of signs and symptoms of monkeypox for 21 days from the most recent exposure. In a scenario where signs or symptoms arise in suspected contact, monkeypox testing should be done [Table 2]. Health professionals who are exposed to patients of monkeypox or contaminated objects do not require work restriction if asymptomatic.16
Vaccination
The smallpox vaccine provides cross-immunity against monkeypox. Both pre- and post-exposure prophylaxis with the smallpox vaccine helps in reducing the severity if given to individuals at risk of acquiring infection (laboratory workers and individuals exposed to a confirmed case of monkeypox). The only vaccine licensed for the prevention of smallpox and monkeypox by the US Food and Drug Administration (US-FDA) is JYNNEOS (JYNNEOS is a live attenuated vaccine (two doses 4 weeks apart; subcutaneous) that induce cellular and humoral immunity against smallpox and monkeypox.). If given within 4 days of exposure to a case of monkeypox it may prevent the onset of the disease. However, administration up to 14 days after exposure may decrease its severity. Vaccination after 14 days may be considered in high-risk individuals. It is administered as a subcutaneous injection, two doses, 28 days apart as post-exposure prophylaxis.10 Currently, vaccines for monkeypox are not available in India.
Treatment
Treatment involves supportive management of symptoms and complications. Antiviral agents used in the treatment of smallpox (tecovirimat; DNA polymerase inhibitor and brincidofovir; intracellular viral release inhibitor) and cytomegalovirus (cidofovir; viral DNA polymerase inhibitor) may be recommended in cases with severe life-threatening illness/complications or at threat of severe illness (age <8 years, pregnant or lactating women and immunocompromised individuals), and special site involvement like ocular or anogenital mucosal involvement. Role of intravenous vaccinia immune globulin (VIG-IV) in the management of monkeypox is limited. However, VIG-IV may be recommended in patients with severe disease or for prophylactic use in individuals with severe T cell immunodeficiency who are exposed to the monkeypox virus where smallpox vaccination is contraindicated.22
To conclude, increased awareness amongst dermatologists about the clinical manifestations of monkeypox will enable early recognition and prompt management. Additionally, in-depth knowledge of the steps of sample collection and specimen transportation will increase the probability of establishing an accurate diagnosis. As the monkeypox outbreak evolves, close surveillance to recognise the new trends in the epidemiology of the disease, and regional efforts to improve the availability of treatment options and vaccinations are some of the anticipated challenges in the near future.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest
Financial support and sponsorship
Nil
References
- Unexpected sudden rise of human monkeypox cases in multiple non-endemic countries amid COVID-19 pandemic and salient counteracting strategies: Another potential global threat? Int J Surg. 2022;103:106705.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front Public Health. 2018;6:241.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- 2022. StatPearls [Internet]. Treasure Island (FL):: StatPearls Publishing; In: Available from:https://www.ncbi.nlm.nih.gov/books/NBK574519/
- [Google Scholar]
- A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-7.
- [PubMed] [PubMed Central] [Google Scholar]
- WHO Director-General Declares the Ongoing Monkeypox Outbreak a Public Health Emergency of International Concern. (23 July 2022) Available from:https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern
- [Google Scholar]
- Monkeypox Outbreak Global Map. 2022. Available from:https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- [Google Scholar]
- Infection Prevention and Control of Monkeypox in Healthcare Settings. 2022. Available at:https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html#anchor_1653508896553
- [Google Scholar]
- A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13:e0007791.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Considerations for Monkeypox Vaccination. 2022. Available from:https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
- [Google Scholar]
- The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17:643-50.
- [CrossRef] [PubMed] [Google Scholar]
- Monkeypox virus infection in humans across 16 countries - April-June 2022. N Engl J Med. 2022;387:679-91.
- [CrossRef] [PubMed] [Google Scholar]
- Multistate outbreak of monkeypox—Illinois, Indiana, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:537-40.
- [PubMed] [Google Scholar]
- Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect Dis. 2022;22:1153-62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Monkeypox outbreak in Spain: Clinical and epidemiological findings in a prospective cross-sectional study of 185 cases. Br J Dermatol 2022
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for Management of Monkeypox Disease. Available from:https://main.mohfw.gov.in/sites/default/files/Guidelines%20for%20Management%20of%20Monkeypox%20Disease
- [Google Scholar]
- Syphilis: Review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev. 1999;12:187-209.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical characteristics and outcomes in a population with disseminated herpes zoster: A retrospective cohort study. Actas Dermosifiliogr. 2017;108:145-152.
- [CrossRef] [PubMed] [Google Scholar]
- Disseminated primary herpes simplex virus type 2 infection in a 22-year-old male. Open Forum Infect Dis. 2015;2:ofv092.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- 2022. StatPearls [Internet]. Treasure Island (FL):: StatPearls Publishing; In: Available from:https://www.ncbi.nlm.nih.gov/books/NBK448191/
- [Google Scholar]
- Current outbreak of monkeypox—Essentials for the dermatologist. J Am Acad Dermatol. 2022;87:e171-74.
- [CrossRef] [Google Scholar]
- Treatment Information for Healthcare Professionals. 2022. Available from:https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- [Google Scholar]





