Translate this page into:
Qualitative analysis of nailfold capillaries in diabetes and diabetic retinopathy using dermatoscope in patients with coloured skin
Corresponding author: Dr. Varadraj V. Pai, Department of Dermatology, Goa Medical College, Bambolim, Goa, India. docpai@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ahmad S, Pai V V, Ashwathy S, Ghodge R, Shukla P. Qualitative analysis of nailfold capillaries in diabetes and diabetic retinopathy using dermatoscope in patients with coloured skin. Indian J Dermatol Venereol Leprol. 2024;90:139–149. doi: 10.25259/IJDVL_710_2022
Abstract
Introduction
Diabetes mellitus (DM) is associated with significant morbidity and mortality due to vascular complications. Diabetic retinopathy (DR) is the most common microvascular complication of diabetes. Videocapillaroscope has been the predominant tool for nailfold capillary analysis. We aimed at using the commonly available handheld dermatoscope and observed changes in the nailfold capillaries as a part of evaluating diabetic microvascular involvement.
Materials and methods
A cross-sectional observational study involving 262 patients of diabetes mellitus and 150 controls was conducted for nailfold capillaroscopic changes using a hand-held dermatoscope over a period of 1 year.
Results
All the capillaroscopic variables like tortuosity, increased capillary density, neoangiogenesis, microhaemorrhages, avascular areas, crossing and meandering capillaries and receding capillaries were significantly more among diabetic than healthy controls. Patients with diabetic retinopathy had significant nailfold capillaroscopic features as compared to patients without DR (P value < 0.001). Neoangiogenesis, receding capillaries and avascular area were significantly higher in proliferative DR as against nonproliferative DR (P < 0.001). A positive association was found between the duration of DM and HbA1c values and NFC features. A decrease in the visualisation of NFC features were noted with increasing skin tone. The difference was significantly more between Fitzpatrick skin phototypes 4 and 5.
Limitations
The study was limited by its qualitative nature of accessing parameters as precise quantitative assessment of various findings cannot be done by a hand-held dermatoscope.
Conclusion
Nailfold capillaroscopy is a quick, cost-effective screening tool for identifying patients at high risk of DR in patients with skin of colour. NFC findings may mirror DR changes. The qualitative findings of NFC using a hand-held dermatoscope were comparable to other modes of nailfold capillaroscopy.
Keywords
Diabetes
hand-held dermatoscope
nailfold capillaries
Plain Language Summary
Diabetes mellitus is associated with significant vascular involvement which can even involve the nail folds. This nail fold capillary analysis is usually done with a videocapillaroscope. We aimed at using the commonly available handheld dermatoscope and observing changes in the nailfold capillaries in Indian patients.
The results showed that capillaroscopic findings like tortuosity, increased capillary density, neoangiogenesis, microhaemorrhages, avascular areas, crossing and meandering capillaries, receding capillaries were significantly more among diabetic than healthy individuals. Some of these findings were significantly increased proportionally with respect to severity and duration of Diabetes. Also we found that the nail features were less evident as the skin tone darkens. We concluded that Nail fold capillaroscopy is a quick, cost-effective screening tool in identifying patients at high risk of developing vascular complications in Diabetes among patients with skin of colour.
Introduction
Microvascular involvement is one of the characteristic features of many diseases in dermatology like systemic sclerosis, systemic lupus erythematosus (SLE), Raynaud’s phenomenon, dermatomyositis, psoriasis, diabetes, etc. Diabetes mellitus (DM) is a group of chronic diseases characterised by hyperglycaemia and is associated with significant complications.1,2 Vascular involvement is the major cause of morbidity and mortality in diabetic patients. Both macro and microvasculature changes occur in this disease by changes in capillary flow or permeability, which is associated with basement membrane thickening.1 , 3 The predominant macrovascular complications are coronary artery disease, peripheral arterial disease, stroke, etc. and microvascular complications are diabetic retinopathy, nephropathy and neuropathy.2
Diabetic retinopathy (DR) is considered to be the most common microvascular complication of diabetes.4 In the US, approximately 10,000 new cases of blindness are due to DR every year.4 The number of patients with DR in America is estimated to reach 16 million by 2050, with vision-threatening complications affecting around 3.4 million of them.5 In south India, DR has an estimated urban prevalence of 18.0% and a rural prevalence of 10.3% among patients with diabetes.6 The risk of developing DR or other microvascular complications of diabetes depends on the duration and the severity of hyperglycaemia.2 Microaneurysms, leukocyte adhesion and apoptosis of vascular and neuronal cells are the early changes of DR. Capillary degeneration and development of acellular capillaries cause a reduction in capillary perfusion and hypoxia which results in neovascularisation which is a characteristic feature of proliferative diabetic retinopathy (PDR).7 Also diabetic retinopathy is an independent predictor of complications such as nephropathy, cardiovascular disease and mortality in patients with diabetes.8
The dermatological study of cutaneous vasculature involves nailfold capillary analysis (capillaroscopy). Nailfold capillaroscopy (NFC) is an easy, non-invasive and useful diagnostic tool to evaluate the microvascular structure of nailfold.7 NFC was initially done for Raynaud’s phenomenon and other connective tissue disorders like systemic sclerosis (SSc), SLE and dermatomyositis. The numerical and morphologic characteristics of vascular parameters to be analysed have undergone many modifications and standardisations over years.9 NFC has recently been incorporated into the new classification criteria of the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2013 for SSc, confirming the importance of the method in the diagnosis of the disease.10 Capillaroscopy has been used to predict the risk of development of complications and digital ulcers in patients with systemic disease.1 The instruments used to observe capillaries have ranged from magnifying glasses to microscopes of various designs and magnifying power.11 The dermatoscope, ophthalmoscope, light microscope (stereomicroscope) and videocapillaroscopes are among the few used for this purpose.9 For evaluating the overall pattern of the capillary bed involvement a total magnification of ×12 to ×14 which offers a wide field of observation and a perception of depth,is ideal but the study of individual capillaries requires a higher magnification capillaroscopy.11
Modern-day capillaroscopy demands the use of specialised equipment which often necessitates an additional investment or a referral to a specialised centre. We aimed at using the commonly available hand-held dermatoscope and observing changes in the nailfold capillaries as a part of evaluating diabetic microvascular involvement. The study intended to determine correlation between nailfold capillaroscopic findings and diabetic retinopathy, and to compare dermatoscopic findings with other studies and changes in patterns among patients of skin of colour.
Materials and Methods
A cross-sectional observational study was conducted among patients attending the dermatology, ophthalmology and medicine outpatient department of a tertiary care centre in Goa, India over a period of 1 year from November 2020 to October 2021. The study was cleared by the hospital’s ethical committee and informed consent was obtained from all participants. A total number of 262 patients with an established diagnosis of diabetes mellitus type 2 and a control group of 150 participants were included in this study for capillaroscopy examination.
The control group consisted of randomly selected healthy subjects among the same age group without any skin disease or systemic disease. People with connective tissue disorders, Raynaud’s phenomenon, pregnancy, systemic diseases causing retinopathy, organ dysfunction (liver, kidney, heart encephalopathy, etc.), history of smoking, hypertension, retinal photocoagulation, nail-biting, prolonged contact with water/detergents and sepsis were excluded from the study group.
A detailed history of diabetes including information on diabetic retinopathy following ophthalmoscopic evaluation was noted. Nailfold capillaroscopy (NFC) was later performed by a dermatologist for all cases and controls. After a rest period of 15–20 minutes at room temperature during which a brief history and clinical examination was done which included noting the skin colour, the ultrasonographic gel was applied to the proximal nailfold of both hands. A dermatoscope (DermLite DL4 dermatoscope) was placed on each proximal nailfold and viewed through the ocular eyepiece. All examinations performed by the authors were cross-checked before finalising the results. NFC was qualitatively analysed with respect to various parameters as mentioned in previous studies by Uyar et al., Bergman et al., Grover et al. and Jakhar et al.7,9,11,12
Abnormal capillaroscopic findings were defined as follows7,9,11–16 [Figures 1 to 3]:
Tortuous capillaries: capillary limbs are curled but do not cross.
Neoangiogenesis includes bushy capillaries (the limb branches present themselves in small and multiple buds), ramified capillaries (abnormal connections between arterial and venous limbs or different capillaries), bizarre capillaries (capillaries with atypical morphology, distinct from other described categories (cloverleaf, musical note G, etc) and coiled and interconnected capillaries with marked heterogeneity in size.
Microhaemorrhages/extravasation: microbleeding around the capillary appears as dark masses adjacent to the distal row.
Avascular areas: loss of two or more consecutive capillaries.
Increased capillary density: capillary density is defined as more than nine capillaries in a 1 mm length of the distal row of each finger or toe.
Mega capillaries/ectasia: the presence of homogeneously and/or irregularly enlarged microvascular loops
Receding capillaries: They are individual capillaries maintaining their position in the distal-most row but presenting slightly proximal to the distal-most loops. They probably gave an impression that the capillary had receded backwards but had yet not dropped out.
Crossing and meandering loops: in crossing capillary, the capillary limbs cross at one point and in meandering capillaries the limbs cross upon themselves or with each other several times

- (a) Tortuous capillaries, (b) bushy capillaries (blue arrow), (c) ramified capillaries (black arrow), (d) bizzare capillaries—musical note G (green arrow) irregularly shaped (blue)
![(e) Avascular areas, (f) mega capillaries (blue) tortuosity (red), (g) microhaemorrhage, (h) increasing capillary density [10x]](/content/126/2024/90/2/img/IJDVL_710_2022_g002.png)
- (e) Avascular areas, (f) mega capillaries (blue) tortuosity (red), (g) microhaemorrhage, (h) increasing capillary density [10x]
![(i) Receding capillaries, (j) crossing capillary (red arrow), meandering capillary (black arrow), (k) crossing capillary (red arrow), mega capillaries, microhaemorrhage (blue) [10x]](/content/126/2024/90/2/img/IJDVL_710_2022_g003.png)
- (i) Receding capillaries, (j) crossing capillary (red arrow), meandering capillary (black arrow), (k) crossing capillary (red arrow), mega capillaries, microhaemorrhage (blue) [10x]
Results
Two hundred and sixty two patients with diabetes mellitus (male = 164, female = 98) and one hundred and fifty controls (male = 86, female = 64) (n = 412) were included in the study. The demographic characteristics of the study population are given in Table 1.
| Baseline characteristics | Baseline characteristics of study groups | P-value | ||
|---|---|---|---|---|
| Controls (n = 150) | Type 2 DM with diabetic retinopathy or DR+ve (n = 118) | Type 2 DM without diabetic retinopathy or DR–ve (n = 144) | ||
| Age ± SD (years) | 50 ± 13 | 56 ± 12 | 53 ± 12 | 0.283 |
| Male:female ratio | 86:64 | 77:41 | 87:57 | 0.418 |
| Mean disease duration ± SD (years) | 0 | 6.9 ± 4.4 | 5 ± 3.8 | <0.001 |
| Mean HbA1c ± SD (%) | 0 | 9.4 ± 2.2 | 7.3 ± 1.7 | <0.001 |
The age group was comparable between patients with diabetic retinopathy (DR+ve) and patients without diabetic retinopathy (DR–ve). Males were the predominant participants in all three groups. The duration of disease was significantly higher in the DR+ve group (6.9 ± 4.4 years) as compared to the DR–ve group (5 ± 3.8 years). The HbA1c value was also significantly higher for the DR+ve group (9.4 ± 2.2 %).
A comparison of NFC findings among patients with DM and controls is shown in Figure 4. Tortuosity, increased capillary density and neoangiogenesis were the three most common findings in the diabetic group. All the capillaroscopic variables were significantly more among diabetic rather than healthy controls (P value <0.001 for all the parameters).
![Comparison of NFC finding among controls, patients with DM, patients with diabetes without retinopathy (DR-ve), patients with Diabetes with retinopathy (DR+ve), Nonproliferative Diabetic retinopathy [NPDR] and Proliferative Diabetic retinopathy [PDR] [N–represents number of participants in each group]](/content/126/2024/90/2/img/IJDVL_710_2022_g004.png)
- Comparison of NFC finding among controls, patients with DM, patients with diabetes without retinopathy (DR-ve), patients with Diabetes with retinopathy (DR+ve), Nonproliferative Diabetic retinopathy [NPDR] and Proliferative Diabetic retinopathy [PDR] [N–represents number of participants in each group]
A comparison of NFC findings among patients with DR–ve and DR+ve is given in Figure 4. Tortuosity, increased capillary density and neoangiogenesis were among the most common finding in both groups. All the capillaroscopic parameters were significantly greater in DR+ve patients as compared to DR–ve patients (P value <0.001). Evaluation of diabetic severity in comparison with the significant capillaroscopic finding using the receiver operator characteristic (ROC) curve is shown in Figure 5. The area under the curve (AUC) for significant NFC findings like tortuosity (0.766), increased capillary density (0.780), neoangiogenesis (0.724) and cross/meandering capillary (0.728) were between 0.7 and 0.8 with ROC analysis. Increased capillary density was the best parameter for DR detection.
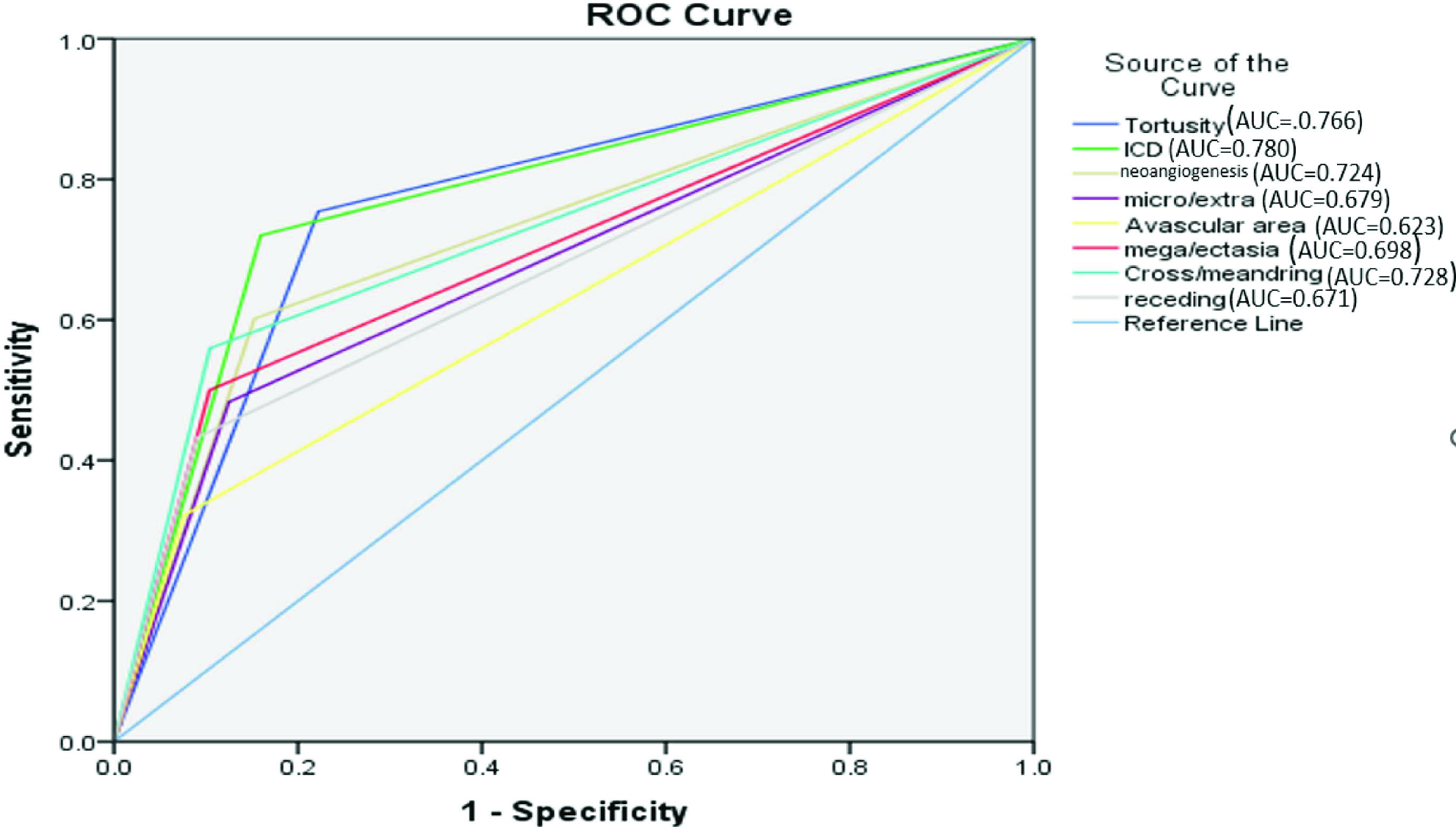
- ROC of significant capillaroscopic findings for DR detection.
A comparison of NFC findings among patients with nonproliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) is given in Figure 4. The P values of the following parameters were significant: neoangiogenesis (P value <0.001), receding capillaries (P value 0.027) and avascular area (P value <0.001). Evaluation of the performance of the significant capillaroscopic finding to note the severity of DR detection using the ROC curve is given in Figure 6. The area under the curve (AUC) for the tortuosity (0.720), neoangiogenesis (0.744), microhaemorrhages (0.714), avascular areas (0.800), cross/meandering (0.735) and receding capillaries (0.736) was between 0.7 and 0.8 with receiver operator characteristic (ROC) analysis. The avascular area was the best predictor of proliferative DR detection.
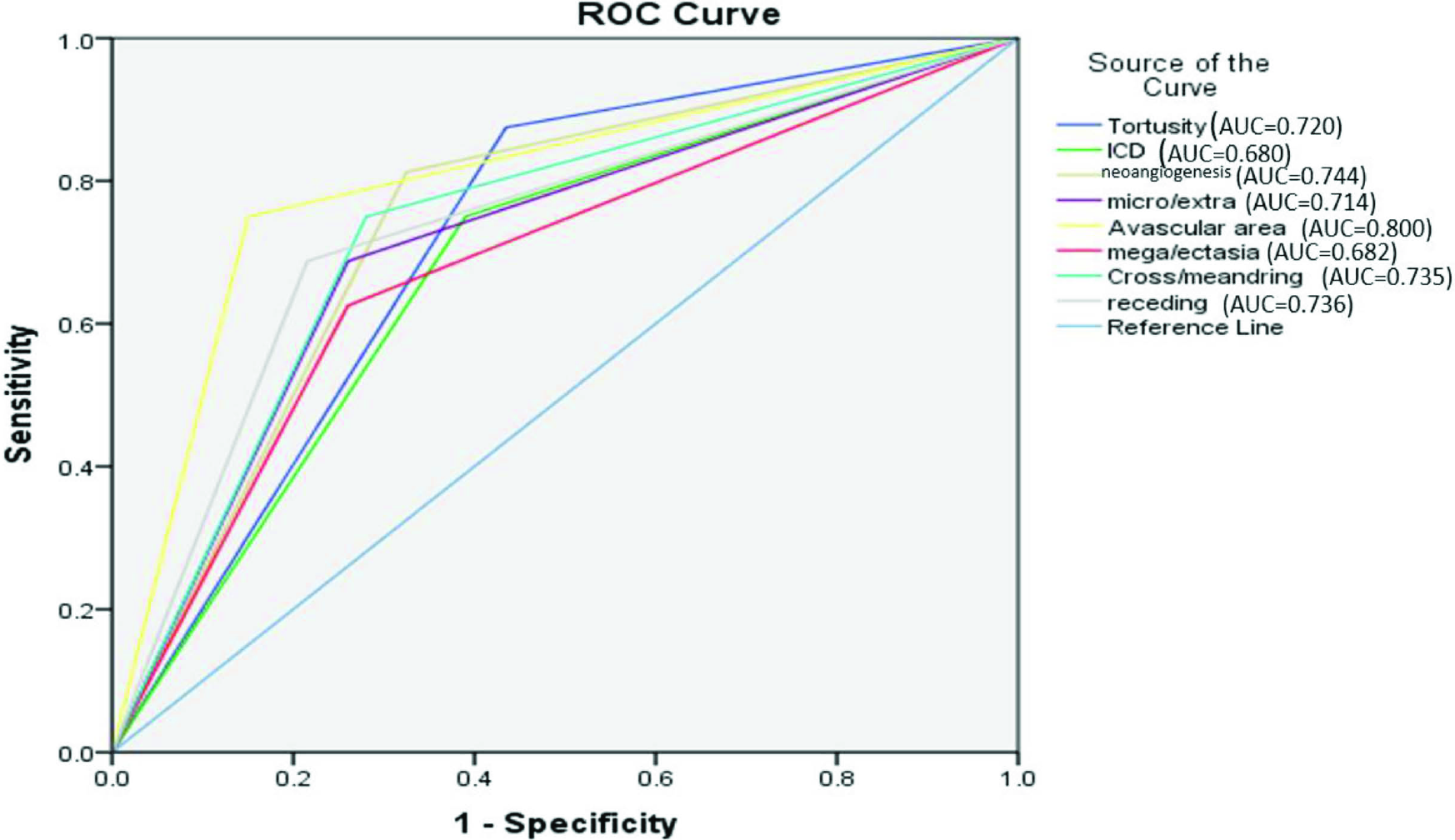
- ROC of significant parameters to differentiate PDR from NPDR
A comparison of the duration of diabetes with NFC features and DR is given in Figure 7. The median age of all NFC features was seen in patients with a history of DM of more than 5 years of duration The duration of diabetes was directly proportional to the increased chances of NPDR and PDR and also to the changes in the nailfold capillaries. Proliferative DR was noted at a median age of 10 years of detection of DM.
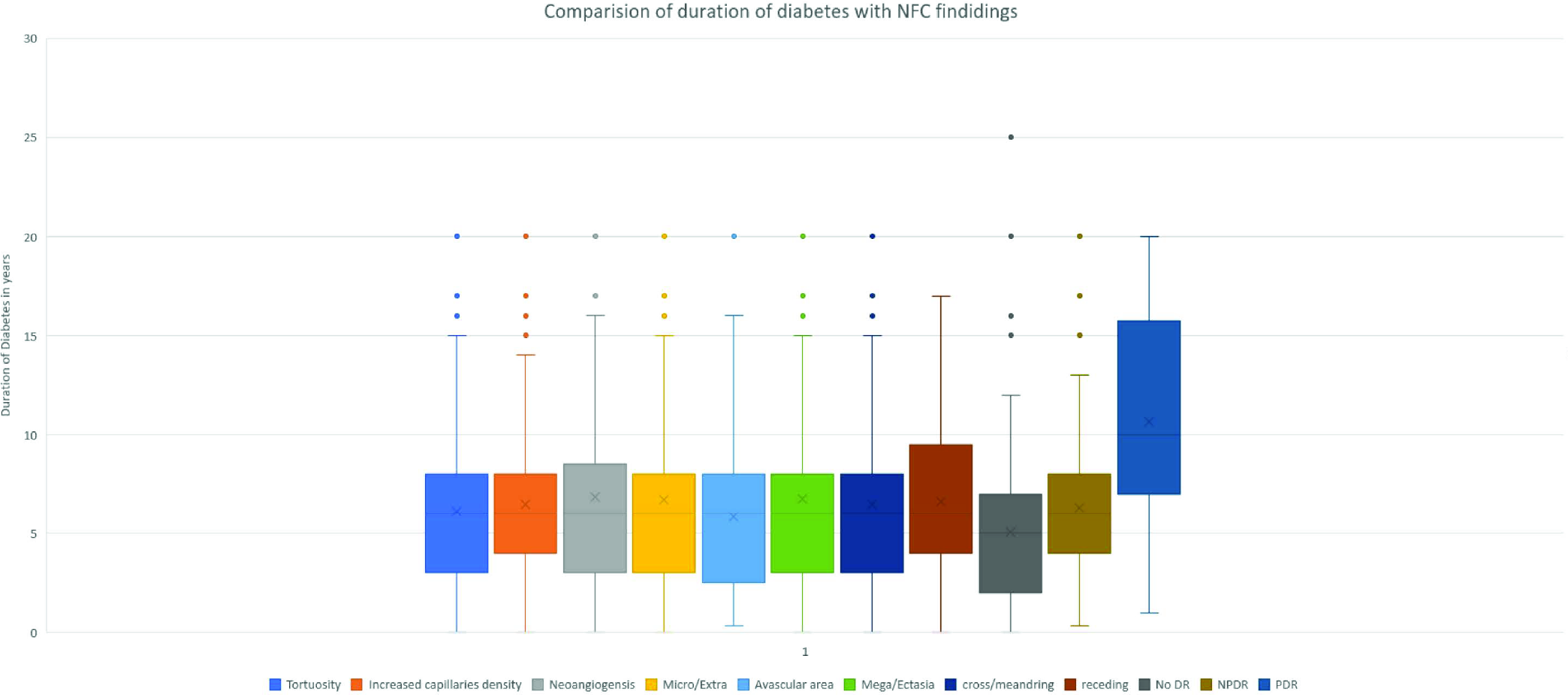
- Comparison of duration of diabetes with NFC findings and DR severity
A comparison of HbA1c values with DR and capillaroscopic findings are given in Figure 8. Most capillaroscopic findings were seen after an HbA1c value of 8%. NFC parameters like extravasation, meandering and ramification had a median value of more than 10%. Well-controlled DM was less frequently associated with DR with a mean value of 7.3% and median value of HbA1c of 7.1%. PDR had a higher median value than NPDR (9.7% and 9%, respectively) and an upper quartile value of HbA1c >10.
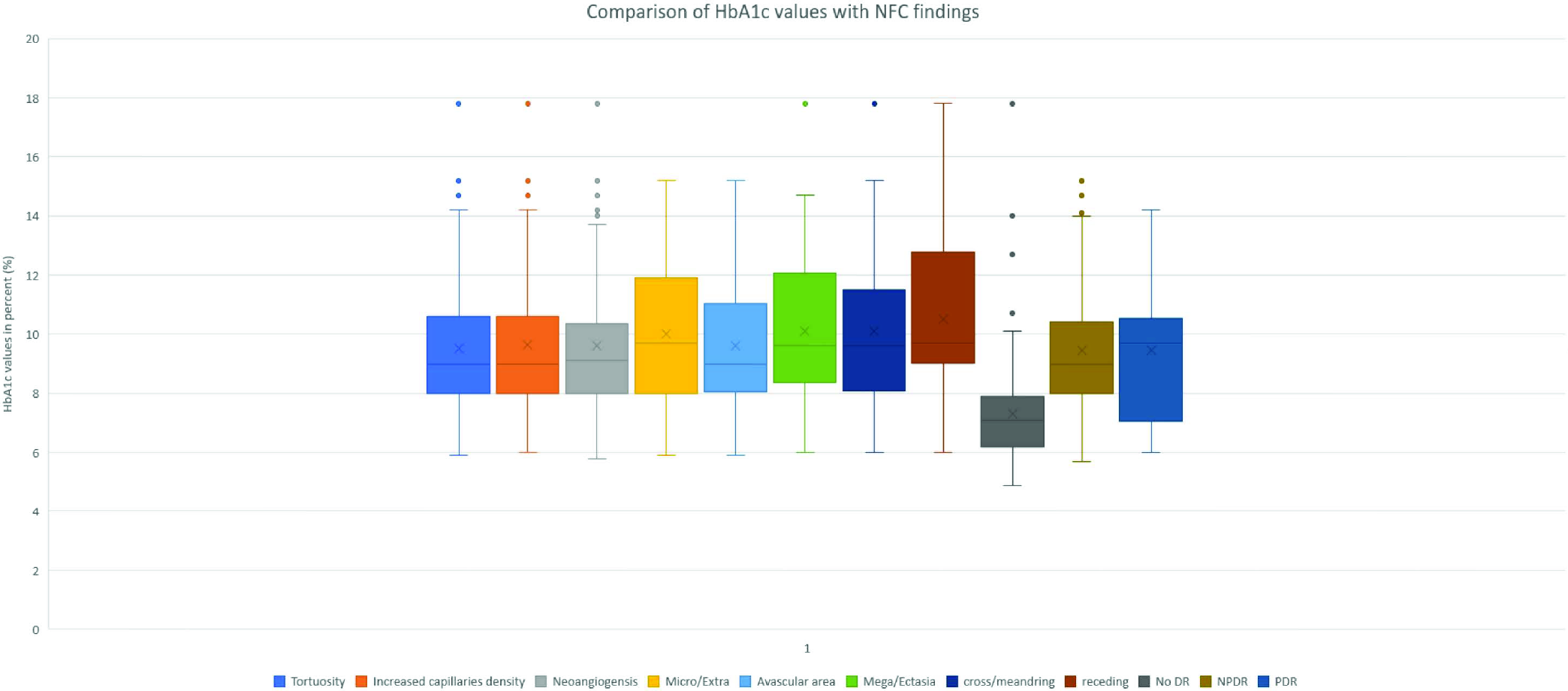
- Comparison of HbA1c values with NFC findings and DR severity
A comparison of NFC patterns with the skin colour of the patients are given in Figure 9. Most patients (80%) belonged to Fitzpatrick skin phototypes 4 (n = 118) and 5 (n = 92). The remaining patients had skin type 3 (n = 41) and skin type 6 (n = 11), respectively. A decrease in the visualisation of NFC features were noted with increasing skin tone. The changes were comparable between skin types 3 and 4. The difference was significantly more between skin types 4 and 5.
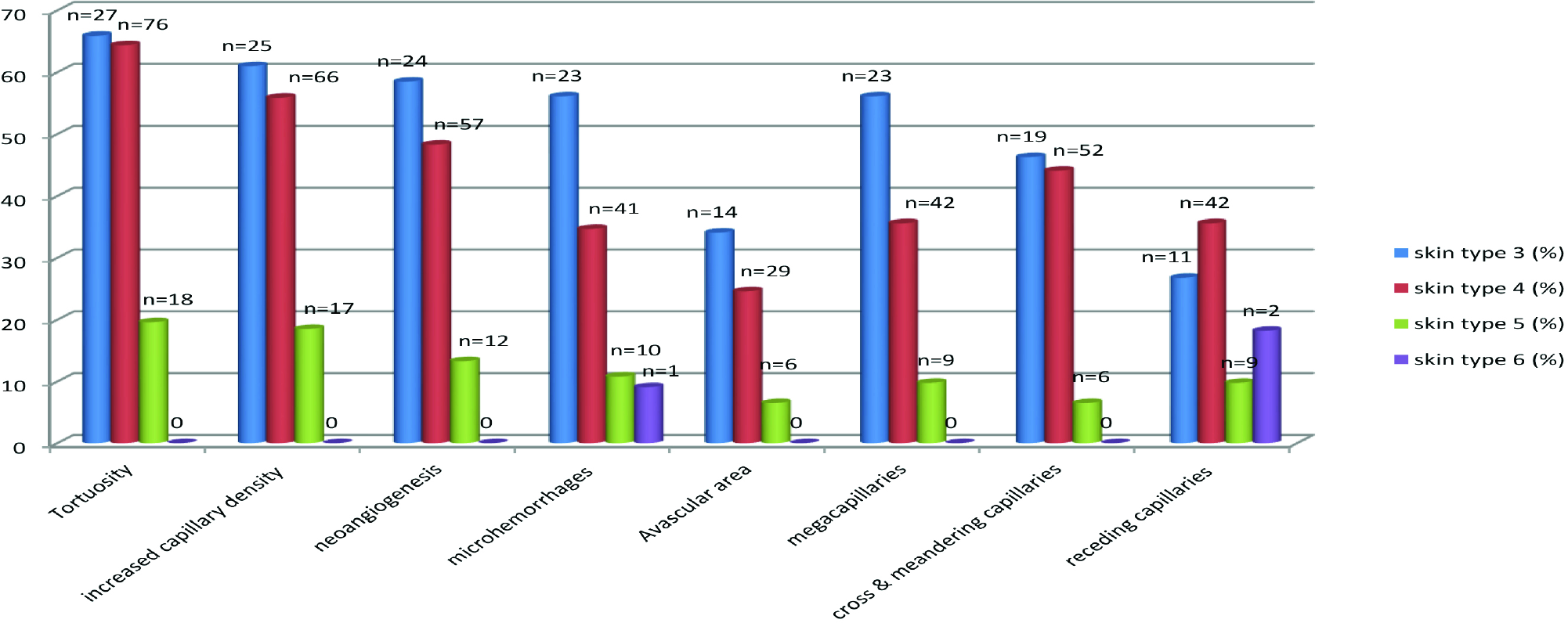
- Comparing NFC findings with skin type of patients
Discussion
The prevalence of diabetes mellitus (DM) in India and worldwide has been steadily increasing. DM is associated with significant morbidity and mortality due to vascular complications. Diabetic retinopathy (DR) is the most common microvascular complication of diabetes and remains a major cause of preventable blindness.7,17,18 The majority of patients with DR have no symptoms until an advanced stage and are known to rapidly deteriorate. Early diagnosis through annual screening for DR, and appropriate treatment can reduce the risk of blindness.17 The vascular changes in the fundus can be observed directly by a noninvasive ophthalmoscope. Ophthalmoscopy alone may not detect all cases of diabetic retinopathy because of its limitation in detecting lesions at the capillary level. The nailfold provides another field through which noninvasive capillaroscopy can be used to monitor vascular changes in the capillaries.19
Studies on NFC changes and diabetes have predominantly used video capillaroscope or USB dermatoscope in addition to the dermatoscope and ophthalmoscopes to analyse changes in the microvasculature in diabetes.1,12 In ophthalmoscopy, the field is too small for proper evaluation. While a video dermoscope allows a precise measurement of capillaroscopic parameters it comes at a high cost and there is loss of panoramic view of the nail. The hand-held dermatoscope has a wide field of view and provides a straightforward, rapid and low-cost procedure with a device that many dermatologists already own.11,20,21 Table 2 compares the dermatoscopy features of NFC parameters in our study with other studies which have used video capillaroscopy. The results show that all major qualitative findings of capillaroscopy can be seen on hand-held dermoscopic examination. Also, qualitative features can be appreciated with almost similar frequency as compared to video capillaroscopy but further quantitative analysis requires a dermatoscope of higher magnification.7,22–26
| Present study | Uyar et al. | Maldonado et al. | Mohanty et al. | Lisco et al. | Kaminska-Winciorek et al. | Sibel Bakirci et al. | |
|---|---|---|---|---|---|---|---|
| Dermatocope (DermLite DL4 dermatoscope) | Video cappilaroscopy (Videocap, DS MediGroup, Milan, Italy) | Stereo microscopes with magnification of 10–100× (Dino-Lite) | Video cappilaroscopy (Jiangsu Jiahua, JH 1004, China) | Picocap Microlab Electronica® (Padua), ×300 magnification | Fotofinder Teach Screen Software videodermatoscope equipped with a capillary probe | Dino-Lite capillaroscopy device (with Dinocapture 2.0 windows software) (magnification ×500) | |
| Tortuosity | 46.1% | 67.5% | 63% | 52.8% | 84% | 10% | 79.5% |
| Neoangiogenesis (Bushy+Ramification+Bizarre) | 35.4% | 75.4% | 11% | 10.4% | 65.1% | 11% | 18.1% |
| Microhaemorrhage/extravasation | 28.6% | 10.6% | − | 28.4% | − | − | 29.5% |
| Mega capillaries/ectasia | 28.2% | 8.3% | − | − | 63.8% | 27% | 95.4% |
| Avascular area | 18.7% | 1.3% | 48% | 28.4% | 60% | − | 0% |
| Cross/meandering | 30.9% | 3.2% | 59% | − | − | 18% | 97.7% |
| Receding capillaries | 24.4% | − | − | − | − | − | |
| Increased capillaries density | 41.2 | − | − | − | − | 69% | − |
In the present study, we found tortuosity, increased capillary density, neoangiogenesis, microhaemorrhage, avascular area, mega capillaries, crossing/meandering capillaries and receding capillaries to be significantly higher in patients with type 2 DM than healthy controls. Similar studies by Uyar et al. and Lisco et al. reported that capillaroscopic findings (tortuosity, bushy capillaries and neoangiogenesis bizarre capillaries) were significantly higher in patients with DM than in healthy controls.7,24 Barchetta et al. performed NFC, ophthalmoscopy, and retinal fluorangiography in all subjects (49 patients and 39 controls) and used quantitative evaluation of nail videocapillaroscopy and score. The authors found that increased density, irregular length and distribution of capillary loss, aberrant morphological alterations such as tortuosity, ramifications and bushes, presence of exudates, oedema and flux abnormalities were detected by NFC in diabetic patients. Moreover, according to Barchetta et al.’s study, NFC was capable of identifying alterations in almost 50% of patients with diabetes without retinopathy.27 In our study, the NFC findings in DM without retinopathy (DR–ve) were noted but in significantly lower numbers [Figure 4].
In our study, a significant difference was noted in all parameters between DM patients without retinopathy (DR–ve) and those with retinopathy (DR+ve), in relation to tortuosity, increased capillary density, neoangiogenesis, microhaemorrhage, avascular area, mega capillaries, crossing/meandering capillaries and receding capillaries. A study by Shikama et al. showed a higher percentage of crossing capillaries in the finger nailfold,being associated with DR in patients with type 2 diabetes mellitus. However, the definition used for crossing capillaries in the above study juxtaposed with our definition of both crossing capillary and meandering loops. The study described that the association of crossing capillaries and DR was independent of traditional risk factors and inhibiting factors for DR like age, sex, duration of diabetes, HbA1c, SBP, BMI and the use of RAS inhibitors and antilipidemic medication.17 In our study, increased capillary density and tortuosity were the best parameters for DR severity as detected by their AUC values of 0.780 and 0.766. Crossing capillaries were seen in 58.5% with DR and had an AUC value of 0.728 indicating good sensitivity. All the parameters were significantly increased in DR+ve patients as compared to DR–ve patients. The distribution in positivity noted in DR–ve patients was similar to DR+ve patients with respect to tortuosity, increased capillary density and neoangiogenesis, which represented the most common features in both groups. These findings indicate that these nailfold capillaroscopic features may precede DR and can predict the risk of the development of DR.
The cause for such changes is not known but is believed to be a common pathological pathway associated with the loss of pericytes that produces both the finger crossing capillaries and DR. Pericyte loss is believed to be an early hallmark of diabetes-associated microvascular disease, including retinopathy and nephropathy.17
Proliferative DR is associated with generalised microangiopathy that affects the skin and is also associated with cerebral bleeding.28 In our study we found that nailfold capillaroscopic findings increase with the severity of retinopathy, i.e., proliferative DR is associated with increased changes in nailfold capillaries than non-proliferative DR. The differences were more pronounced among neoangiogenesis, avascular areas and receding capillaries variables. The area under the curve (AUC) for the avascular area was 0.80 with receiver operator characteristic (ROC) analysis indicating a good sensitivity. A study by Mohanty et al. comparing 66 patients of NPDR and 59 patients of PDR found tortuosity, neoangiogenesis, microhaemorrhages, avascular areas and reduced nailfold capillary density significantly increases in proliferative diabetic retinopathy.23
The pathogenetic mechanisms of microangiopathy in diabetic retinopathy and diabetic dermopathy are related and include excess sorbitol formation, increased glycation end products, oxidative damage and protein kinase C overactivity. The high blood glucose levels over a prolonged period damage the retinal microvasculature and result in ischaemia. In response, factors such as vascular endothelial growth factors are released from the ischaemic retina, inducing the formation of new blood vessels. These new blood vessels are often fragile and prone to leaking. The natural course of PDR involves highly active phases of retinal neovascularisation and fibrous proliferation, potentially leading to visual loss if left untreated.29 The NFC features probably reflect a similar pathogenic mechanism.
The propensity of developing diabetic retinopathy is directly proportional to the age of the patient and duration of diabetes as well as with poor glycaemic control.30 The prevalence of retinopathy in dependence with the duration of diabetes was 1.1% at diagnosis, 6.6% after 0<5 years, 12% after 5<10 years, 24% after 10<15 years and 63% after ≥30 years.31 Our study also found that the duration of diabetes increases the chances of retinopathy in patients. Our study of the NFC structures also described similar results with all the parameters appearing at a median duration of 5 years after detection of diabetes [Figure 7]. Kaminska-Winciorek et al. analysed the NFC among children with type 1 DM and found an increase in the number of capillaries, corresponding to the duration of diabetes, especially in older children, most frequent in girls. The study showed that abnormal capillaries were seen on NFC after a median duration of approximately 5 years as seen in our study.25 A study by Kuryliszyn et al. revealed no correlation between changes in capillaroscopic images and age, BMI and the time course of diabetes. But spiral capillaries with a tendency of shortening and enlargement together with a pink background accompanied by neovascularisation was found in the cases of advanced diabetic microangiopathy.32
Glycosylated haemoglobin (HbA1c), which reflects the chronic blood glucose concentration, is a reliable marker of possible diabetes and predicts cardiovascular disease and mortality. Cho et al. published that the optimal HbA1c cut-off for detecting any diabetic retinopathy was 6.6%, and for moderate or severe retinopathy was 6.9%. Our study also found that the mean HbA1c value was 9.4 ± 2.2 in DR+ve patients and increasing values of HbA1c was associated with the severity of DR [Figure 8]. Most capillaroscopic results were seen after an HbA1c value of 7.5%. NFC parameters like extravasation, meandering and ramification had a median value of more than 10%. Similar findings were seen in a study by Kaminska-Winciorek et al. where the higher values of HbA1c influenced the capillaroscopic images, mainly the number of vessels.25 Kuryliszyn et al. also found that a level of HbA1c above 6.1% was found in more than 90.9% of patients with advanced capillary changes.32 Hosking et al. demonstrated a positive association between high HbA1c and the number of microhaemorrhages.33
Dermatoscopy in the skin of colour is affected by the presence of high melanin content which prevents the visualisation of vessels in NFC.34 Our study corroborated this observation. We found that an increase in skin colour significantly hampered the visualisation of NFC. The findings were comparable between skin colours 3 and 4 but significantly decreased with skin colours 5 and 6 [Figure 9]. A study by Weekenstroo et al. suggested that as compared to white light, a green light may provide better visualisation of the NFC.35 Also the use of yellow or red light with multispectral dermatoscopes may be useful in offering a better contrast in dark skin individuals.34
Limitations
The study was limited by its qualitative nature of accessing parameters as precise quantitative assessment of various findings cannot be done by a hand-held dermatoscope. Also, the time gap between ophthalmoscopic evaluation and NFC study may affect the correlation of features.
Conclusion
Diabetic retinopathy is an independent predictor of complications such as nephropathy, cardiovascular disease and mortality in patients with diabetes. Nailfold capillaroscopy is a quick, cost-effective screening tool in identifying patients with higher risk for DR and other microangiopathic complications of diabetic mellitus. Our study demonstrated that not only the severity of the changes in capillaries increases with increasing severity of diabetic retinopathy but also that microangiopathy at the NFC may appear before the clinical diagnosis of diabetic retinopathy. The qualitative findings of NFC using a hand-held dermatoscope were comparable to other modes of nailfold capillaroscopy. The evaluation of nailfold capillaroscopic findings can be one of the modalities for vascular assessment of diabetic patients to diagnose and follow-up microvascular complications.
Acknowledgment
The authors would like to thank the help provided by the department of Ophthalmology, Goa Medical College.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Nailfold capillaroscopy findings in diabetic patients (a pilot cross-sectional study) Open J Pathol. 2015;5:65-72.
- [CrossRef] [Google Scholar]
- Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77-82.
- [CrossRef] [Google Scholar]
- Diabetic microvascular disease: An Endocrine Society scientific statement. J Clin Endocrinol Metab. 2017;102:4343-410.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence of diabetic retinopathy in India: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study report 2. Ophthalmology. 2009;116:311-8.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of the relationship between diabetic retinopathy and nailfold capillaries in type 2 diabetics with a noninvasive method: Nailfold videocapillaroscopy. J Diabetes Res. 2016;2016:7592402.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Optimal HbA1c cutoff for detecting diabetic retinopathy. Acta Diabetol. 2013;50:837-42.
- [CrossRef] [PubMed] [Google Scholar]
- Nail-fold capillaroscopy for the dermatologists. Indian J Dermatol Venereol Leprol. 2022;88:300-12.
- [CrossRef] [PubMed] [Google Scholar]
- 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2013;72:1747-55.
- [CrossRef] [PubMed] [Google Scholar]
- The handheld dermatoscope as a nail-fold capillaroscopic instrument. Arch Dermatol. 2003;139:1027-30.
- [CrossRef] [PubMed] [Google Scholar]
- Nail fold capillaroscopic changes in patients with type 2 diabetes mellitus: An observational, comparative study. Indian J Med Spec. 2020;11:28-33.
- [CrossRef] [Google Scholar]
- The impact of nailfold capillaroscopy in the approach of microcirculation. In: Vascular Biology—Selection of Mechanisms and Clinical Applications. Marcelo: IntechOpen; 2019. [as accessed on 2022 Jan 18]. Available from:
- [Google Scholar]
- Nailfold capillaroscopy in rheumatic diseases: Which parameters should be evaluated? Biomed Res Int. 2015;2015:974530.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Qualitative and quantitative assessment of nailfold capillaries by capillaroscopy in healthy volunteers. Vasa. 2012;41:19-26.
- [CrossRef] [PubMed] [Google Scholar]
- Association of crossing capillaries in the finger nailfold with diabetic retinopathy in type 2 diabetes mellitus. J Diabetes Investig. 2021;12:1007-14.
- [CrossRef] [PubMed Central] [Google Scholar]
- The increasing burden of diabetes and variations among the states of India: The Global Burden of Disease Study 1990–2016. Lancet Glob Health. 2018;6:e1352-62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Use of dynamic capillaroscopy for studying cutaneous microcirculation in patients with diabetes mellitus. Microvasc Res. 1997;53:121-7.
- [CrossRef] [PubMed] [Google Scholar]
- Capilaroscopia en esclerosis sistémica: Una revisión narrativa de la literatura. Rev Colomb Reumatol. 2016;23:250-8.
- [CrossRef] [Google Scholar]
- Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmun Rev. 2020;19:102458.
- [CrossRef] [PubMed] [Google Scholar]
- Nailfold capillaroscopy in diabetes mellitus. Microvasc Res. 2017;112:41-6.
- [CrossRef] [PubMed] [Google Scholar]
- Can nailfold capillaroscopy be a screening tool for diabetic retinopathy—A hospital based cross-sectional study in Orissa, India. J Evid Based Med Healthc. 2021;8:1479-83.
- [CrossRef] [Google Scholar]
- Computerized video-capillaroscopy alteration related to diabetes mellitus and its complications. Adv Exp Med Biol. 2018;1072:363-8.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetic microangiopathy in capillaroscopic examination of juveniles with diabetes type 1. ostepy Hig Med Dosw (Online). 2012;66:51-9.
- [PubMed] [Google Scholar]
- The evaluation of nailfold videocapillaroscopy findings in patients with type 2 diabetes with and without diabetic retinopathy. North Clin Istanb. 2018;6:146-50.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- High prevalence of capillary abnormalities in patients with diabetes and association with retinopathy. Diabet Med. 2011;28:1039-44.
- [CrossRef] [PubMed] [Google Scholar]
- Proliferative retinopathy in type 1 diabetes is associated with cerebral microbleeds, which is part of generalized microangiopathy. Diabetes Care. 2014;37:1165-8.
- [CrossRef] [PubMed] [Google Scholar]
- Proliferative diabetic retinopathy. In: Atlas of Retinal OCT: Optical Coherence Tomography. 2018. p. :88-910.
- [CrossRef] [Google Scholar]
- Diabetic retinopathy [Updated 2021 Aug 29] In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. [as accessed on 2022 Jan 18]. Available from:
- [Google Scholar]
- Prevalence and progression rate of diabetic retinopathy in type 2 diabetes patients in correlation with the duration of diabetes. Exp Clin Endocrinol Diabetes. 2018;126:570-6.
- [CrossRef] [PubMed] [Google Scholar]
- A study on microvascular abnormalities in capillaroscopy in patients with type 1 diabetes mellitus. Diabetol Dośw Klin. 2006;6:98-103.
- [CrossRef] [PubMed] [Google Scholar]
- Non-invasive detection of microvascular changes in a paediatric and adolescent population with type 1 diabetes: A pilot cross-sectional study. BMC Endocr Disord. 2013;13:41.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Yellow light in dermatoscopy and its utility in dermatological disorders. Indian Dermatol Online J. 2017;8:384-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Green light may improve diagnostic accuracy of nailfold capillaroscopy with a simple digital videomicroscope. Rheumatol Int. 2015;35:1069-71.
- [CrossRef] [PubMed] [Google Scholar]







