Translate this page into:
Iatrogenic irritant contact dermatitis to podophyllin and the perils of look-alike, sound-alike trade names in dermatology
Corresponding author: Dr. Kabir Sardana, Department of Dermatology, ABVIMS & Dr RML Hospital, New Delhi, Delhi, India. kabirijdvl@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sardana K, Mathachan SR. Iatrogenic irritant contact dermatitis to podophyllin and the perils of look-alike, sound-alike trade names in dermatology. Indian J Dermatol Venereol Leprol. 2024;90:110-2. doi: 10.25259/IJDVL_72_2023
Dear Editor,
A 45-year-old male presented to the skin outpatient department of Ram Manohar Lohia Hospital, New Delhi with incessant itching and scratching over the left shin. Cutaneous examination revealed hyperpigmentation and thickening with accentuation of skin markings which was consistent with the diagnosis of lichen simplex chronicus. The patient was advised to avoid repeated itching or scratching, and a topical clobetasol propionate 0.05% preparation was administered (powercort-STM) for twice daily application along with tablet hydroxyzine 25 mg at night. One week later, he presented with ulceration, severe burning and pain over the site [Figure 1] with marked hyperpigmentation, consistent with irritant contact dermatitis. The patient was asked to show the medication, and it was found that the patient was applying podophyllin (brand name podowart-S), given by the pharmacist who misinterpreted the drug name (powercort-S vs. podowart-S).1 Notably, podowart-S contains podophyllum resin 25%, benzoin 10% and salicylic acid 5%.
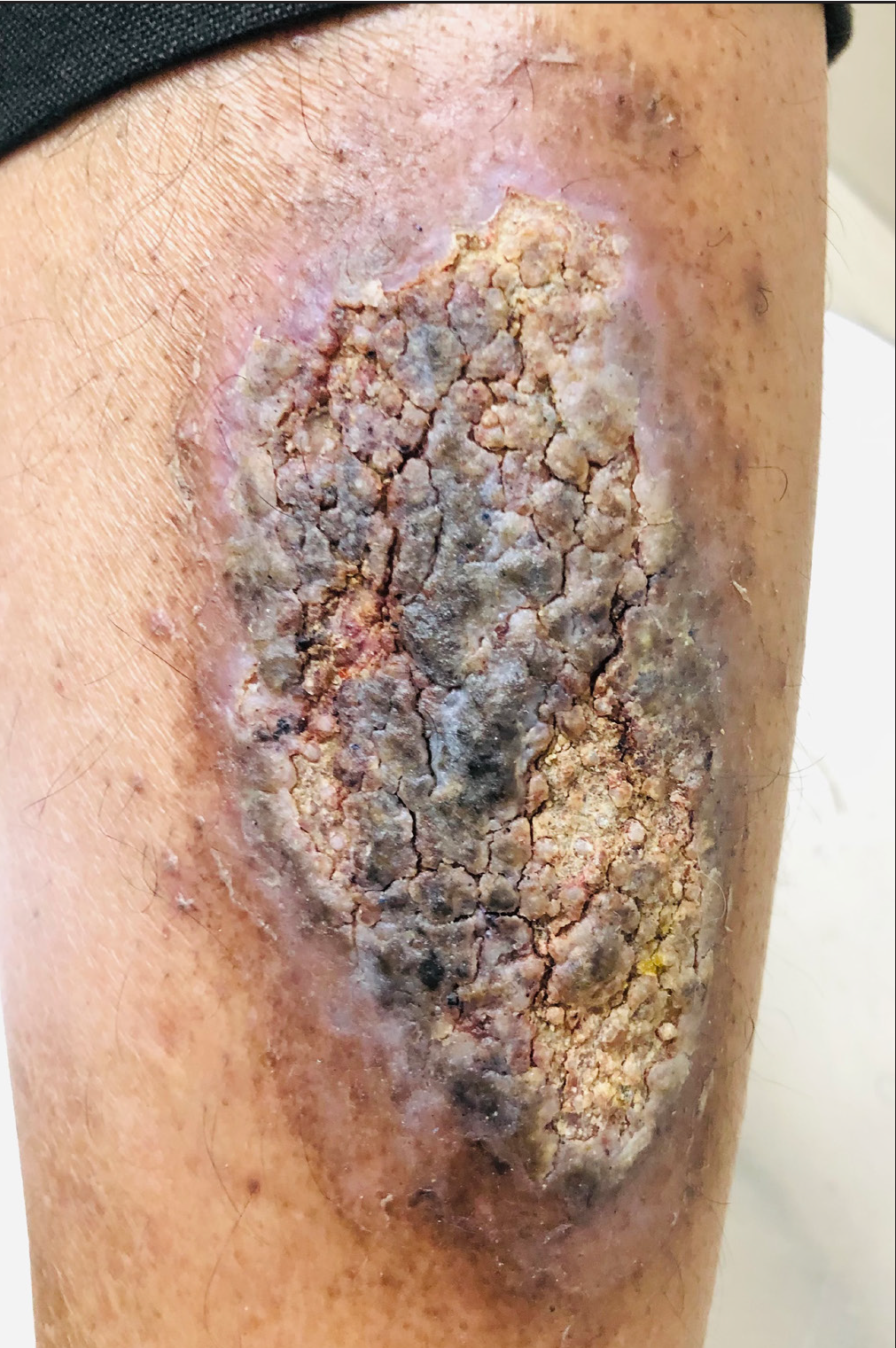
- Irritant contact dermatitis due to the application of podophyllin.
Iatrogenic contact dermatitis is caused by certain sensitizers, including topical antibiotics, antiseptics and corticosteroids, most commonly seen with budesonide, neomycin and benzocaine.2 Our case represented an unusual case of look-alike, sound-alike (LASA) name error, as the prescription was mistaken by the dispensing chemist, which is often the case when a “recent launch” trade name resembles an old drug combination. The LASA errors make up a high proportion of all medication errors (6.2–14.7%)3 and can occur during prescribing, dispensing or administration of medicines. Look-alike, sound-alike errors can result in overdosing, under-dosing or inappropriate dosing.4 The confusion can occur between generic – generic names (e.g., isotretinoin – tretinoin and hydroxyzine – hydralazine); brand – brand names (e.g., prozac – provera); brand – generic names (e.g., soriatane – sertraline); or generic – brand names (e.g., methadone – metadate).5 The cause of the error may lie in similarities in orthography (the written forms) or phonology (the spoken forms).6 Our case represents a brand – brand name error; fortunately, LASA errors are uncommon, seen in 0.00003–0.0022% of prescriptions.7 While such similar names can lead to confusion amongst doctors and pharmacists, the brunt is borne by patients who finally end up using the wrong medications. This can be an issue in India, where there is an alarming trend of over-the-counter drug dispensing and thereby, needs to be notified.
Unlike other forms of medication error (such as wrong patient or wrong route of administration), the onus in case of LASA errors does not fall on the healthcare professionals. The issue of LASA name confusion should be considered to be the responsibility of the manufacturers, regulators, and naming bodies. There is little focus on the error in literature even though there is a clear incentive for pharmaceutical companies to avoid error reduction activities for fear of exposure to liability, regulatory interference and loss of competitive advantage.8 Ideally, manufacturing companies should ensure a thorough background check before naming their product to avoid this problem.9 While the food and drug administration, developed the phonetic and orthographic computer analysis (POCA) tool, which measures the phonetic and orthographic similarities of a proposed brand name against multiple datasets of both brand and generic names, this cannot help retrospectively, as in our case.
Various methods have been proposed to obviate LASA errors, including (i) reducing interruptions and distractions in relation to LASA errors; (ii) typographic adaptation [tall man lettering], (iii) barcoding; and (iv) computerised prescriptions. A simpler and straightforward option is to ensure that the patients countercheck the medicines with their treating doctors, a practice that we often adhere to and this is usually sufficient to avoid such errors.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Podophyllum: an irritant on patch testing. Contact Derm. 1993;29:274-5.
- [CrossRef] [PubMed] [Google Scholar]
- Frequency and trends of contact allergy to and iatrogenic contact dermatitis caused by topical drugs over a 25-year period. Contact Derm. 2016;75:290-302.
- [CrossRef] [PubMed] [Google Scholar]
- Pediatric vaccination errors: application of the “5 rights” framework to a national error reporting database. Vaccine. 2009;27:3890-96.
- [CrossRef] [PubMed] [Google Scholar]
- Medication errors: EMERGing solutions. Br J Clin Pharmacol. 2009;67:589-91.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- ISMP List of Confused Drug Names. ISMP; 2019. Available at: https://www.ismp.org/recommendations/confused-drug-names-list
- Similarity as a risk factor in drug-name confusion errors: the look-alike (orthographic) and sound-alike (phonetic) model. Med Care. 1999;37:1214-25.
- [CrossRef] [PubMed] [Google Scholar]
- The problem of look-alike, sound-alike name errors: Drivers and solutions. Br J Clin Pharmacol. 2021;87:386-94.
- [CrossRef] [PubMed] [Google Scholar]
- Naming, labelling, and packaging of pharmaceuticals. Am J Health Syst Pharm. 2001;58:2033-41.
- [CrossRef] [PubMed] [Google Scholar]
- Medication errors resulting from the confusion of drug names. Expert Opin Drug Saf. 2004;3:167-72.
- [CrossRef] [PubMed] [Google Scholar]





