Translate this page into:
Generalized pustular psoriasis of pregnancy successfully treated with secukinumab
Corresponding author: Dr. Jinbo Chen, Department of Dermatology, Wuhan No. 1 Hospital, Zhongshan Avenue 215, Wuhan, Hubei China. chen999jinbo@163.com
-
Received: ,
Accepted: ,
How to cite this article: Zhang J, Xia P, Wan L, Chen L, Zhou X, Chen J. Generalized pustular psoriasis of pregnancy successfully treated with secukinumab. Indian J Dermatol Venereol Leprol 2023;89:886-8.
Dear Editor,
Generalized pustular psoriasis of pregnancy (GPPP) is a rare and severe condition that threatens the health of the mother and the Foetus. The treatment options for GPPP are limited due to concerns about unfavorable pregnancy outcomes. We report two cases of GPPP successfully treated with secukinumab with no serious adverse events.
The first patient is a 26-year-old female in her 26th week of gestation. She presented with a sudden aggravation of pustular psoriasis in her 20th week of gestation. She was diagnosed case of pustular psoriasis since past 20 years and had received steroids, azathioprine, cyclosporine, acitretin in the past with partial response. The disease flared after discontuation of drugs each time. She was off all medications 6 months prior to the pregnancy. At presentation, physical examination revealed a body temperature of 39℃, brightly erythematous plaques with scattered pustules on the waist, abdomen, back, elbows, and lower limbs [Figures 1a and 1d]. Routine laboratory examinations and serological tests for hepatitis B and interferon-gamma release assays were negative. Ultrasound showed a single live fetus with polyhydramnios. She was diagnosed with GPPP and treated with secukinumab 300 mg subcutaneous injection on day 1 (baseline), followed by secukinumab 150 mg subcutaneous injection on days 8, 15, 22, 29 and 60 for a total of 6 injections. She also received supportive care in the form of rehydration, nutritional support, maintenance of electrolyte balance, antibiotic treatment with azithromycin, and topical emollients. On the third day after admission, the patient was afebrile and most of the pustules started to dry up [Figures 1b and 1e]. By the end of one month, pustules completely disappeared, and erythema subsided [Figures 1c and 1f]. She delivered a healthy baby boy with normal birth weight and normal Apgar score at the 37th week of gestation.
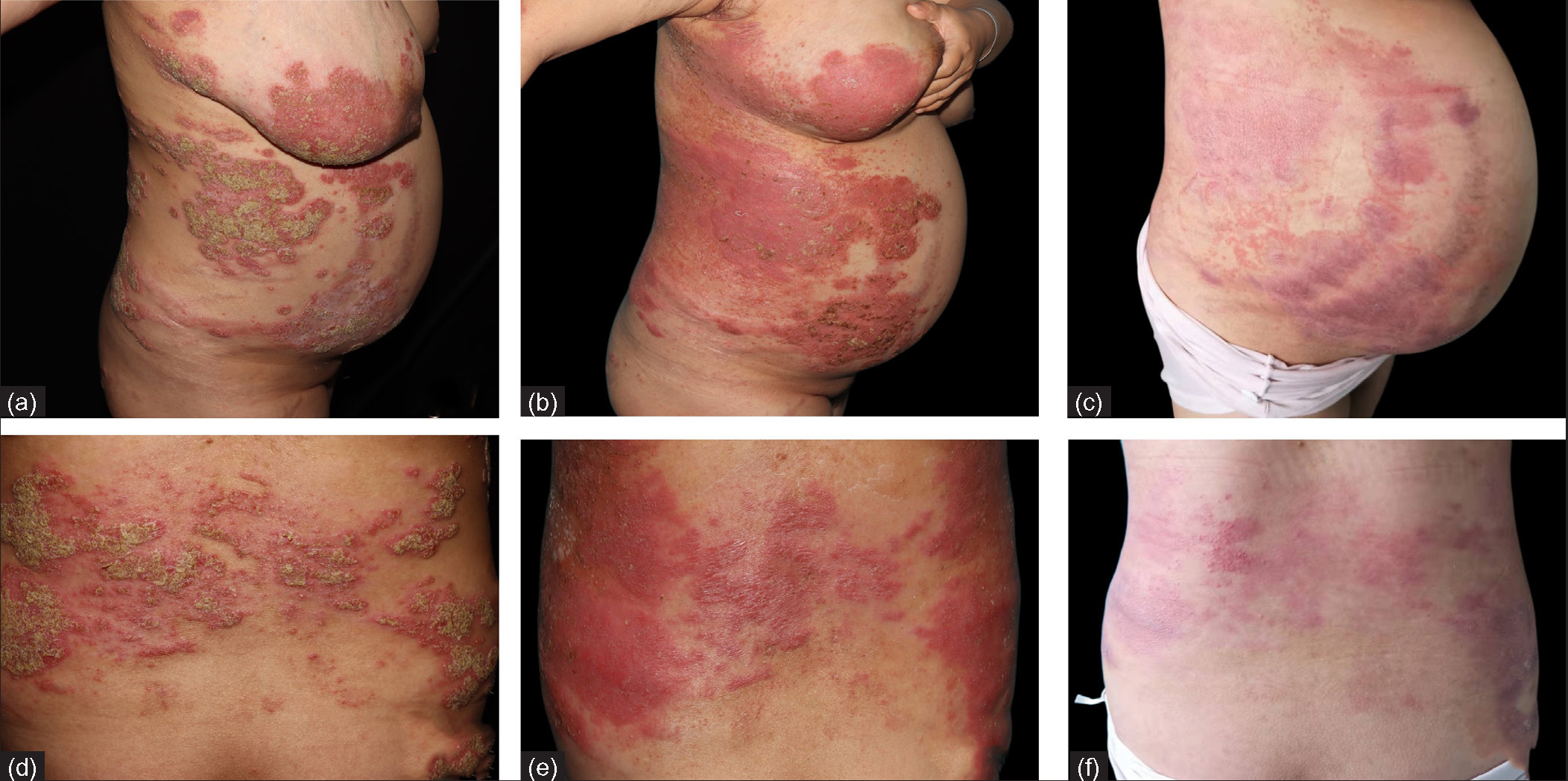
- Clinical images of patient 1 at (a,d) baseline,(b,e) 3 days and (c,f) 28 days after treatment
The second patient, a 27-year-old female was admitted to the hospital at 24 weeks of gestation with a one-month history of pustular flare of preexisting chronic plaque psoriasis which was present since 10 years. She received secukinumab injections for 2 years until she got pregnant, when these were discontinued. At the 20th week of gestation, she presented with a pustular flare over her abdomen and lower limbs without fever. Physical examination revealed multiple erythematous plaques on the trunk and limbs, with extensively distributed tiny pustules, some of which coalesced into lakes of pus [Figures 2a and 2c]. No abnormalities were observed in blood cell counts, liver and renal function, hepatitis B, and tuberculosis screening. Based on her previous good response to secukinumab, subcutaneous injections of secukinumab 150 mg were given at weeks 0, 1, 2 and 4, for a total of 4 injections with which she had a dramatic response. She subsequently delivered a full-term baby boy with a birth weight of 3700 g. At one year follow-up, she was completely clear of active disease and only had post inflammatory hyperpigmentation [Figures 2b and 2d].
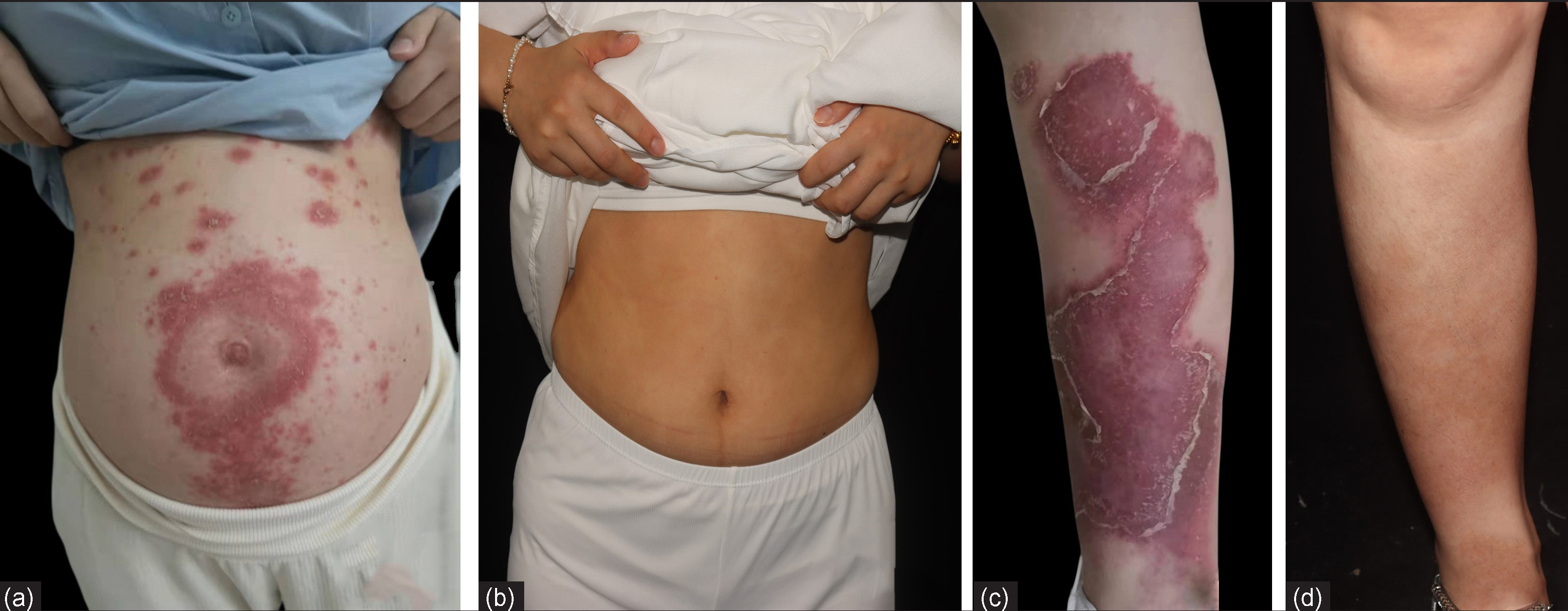
- Clinical images of patient 2 at (a,c) baseline,(b,d) 1 year later after treatment
GPPP is a rare and severe condition that most commonly occurs in the third trimester.1 It is characterized by generalized aseptic pustules on a background of erythema with fever, leukocytosis and increased C-reactive protein. In severe cases, it can evolve into sepsis and endanger maternal and/or fetal life. Treatment with conventional medications, such as methotrexate, acitretin, and cyclosporine are limited by concerns of fetal toxicity. Secukinumab is a human IgG1 monoclonal antibody that selectively binds to and neutralizes interleukin-17A. It has been approved for psoriasis treatment. In contrast to glucocorticoids and cyclosporine, secukinumab has little effect on blood pressure and blood glucose in pregnancy. Data also suggests that the spontaneous abortion rate related to its use was similar to that of the general population. No safety signals were identified regarding spontaneous abortions or congenital malformations. In clinical practice, these data provided reassurance in cases where conception occurs during secukinumab treatment. In practice, only a small number of pregnant women continue treatment throughout their pregnancy, while the majority of them terminate treatment in the third trimester.2,3 In our cases, the patients showed excellent response to secukinumab with favorable maternal and fetal outcomes. It is important to note that when choosing biological therapy during pregnancy, the risk of potential teratogenic effects in the early stages of pregnancy must be considered. In addition, newborns exposed to biologic agents in utero should delay exposure to live vaccines, especially tuberculosis vaccines.4
Larger studies are necessary to confirm the safety and efficacy of secukinumab treatment in GPPP.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Treatment of generalized pustular psoriasis of pregnancy with infliximab. Cutis. 2021;107:2-5.
- [CrossRef] [PubMed Central] [Google Scholar]
- Psoriasis and pregnancy outcomes in biological therapies: a real-life, multi-centre experience. J Eur Acad Dermatol Venereol. 2019;33:374-77.
- [CrossRef] [PubMed] [Google Scholar]
- Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol. 2018;179:1205-207.
- [CrossRef] [PubMed] [Google Scholar]
- The use of biological drugs in psoriasis patients prior to pregnancy, during pregnancy and lactation: a review of current clinical guidelines. Postepy Dermatol Alergol. 2020;37:821-30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





