Translate this page into:
Deciphering the role of vitamin D on skin cancers and tumour microenvironment
Corresponding author: Dr. Priyanka Bhatnagar, Disease Biology Segment, Quick IsCool, Aitele Research LLP, Bihar, India. priyanka.quickiscool@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Nautiyal R, Bhatnagar P. Deciphering the role of vitamin D on skin cancers and tumour microenvironment. Indian J Dermatol Venereol Leprol. 2024;90:192–201. doi: 10.25259/IJDVL_1236_2021
Abstract
Skin cancer is a significant health burden being the fourth most common cancer globally and accounts for 6.2% of the total combined cancer cases. However, mortality rates due to skin cancer are less when compared with other cancers, but it is significantly high in the Asian population (43%). DNA mutations and environmental and genetic factors are linked with skin cancer prognosis; however, long-term exposure to ultraviolet (UV) radiation remains one of the leading factors worldwide. Sun exposure is a major environmental risk factor for skin cancers but is also an essential source of vitamin D. On the other hand, studies exploring the relationship between skin cancer risk and vitamin D show mixed, somewhat conflicting results. This study investigates the role of vitamin D and skin carcinogenesis to clarify the associations. Moreover, in addition to suppressing cancer stem cells, it has been observed that vitamin D also regulates tumour initiation and metastasis. In conclusion, the incorporation of well-designed studies on the metabolism of vitamin D from a genotypic and phenotypic perspective is required to understand the intricate mechanisms linking the role of vitamin D in skin carcinogenesis. These new findings will open up new pathways in targeting the disease and lead to novel opportunities for its treatment and cure.
Keywords
DNA damage
skin cancer
tumour microenvironment
ultraviolet radiation
vitamin D
Skin Cancer
Introduction
Cancer, the emperor of all maladies, has coexisted with humankind since 1600 BC, when the first documented case of breast cancer was found in Edwin Smith Papyrus.1 Since then, the disease has co-evolved with us and has caused a significant burden across the globe. Cancer affects almost every part of the body, including the lungs, breasts, skin, liver, colon and rectum. According to the global cancer statistics in 2020, skin cancer accounts for 6.2% of all new cancer cases.2 The abnormal growth of cells in the epidermis due to mutations, which arise from unrepaired DNA damage, causes skin cancer. Generally, the head and neck are the sites where most cases of skin cancers occur. Skin cancer can be broadly classified into non-melanoma and melanoma as represented in Figure 1. Non-melanoma skin cancers (NMSC) comprise basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). Basal cell carcinoma is generally found in the basal cells of the lower epidermis. Though it grows slowly and rarely spreads to other parts of the body, if left undiagnosed and untreated, it may spread to the surrounding tissues.3

- Classification of skin cancers
On the other hand, squamous cell carcinoma is a type of cancer that develops in the squamous cells. Figure 2 shows the classification of skin cancers and the outermost cell layer of the epidermis.4 Melanomas are cancers that arise in the melanocytes, pigment-producing cells (melanin), present throughout the skin cells.5 Ultraviolet radiations is one of the primary causes of this type of cancer.
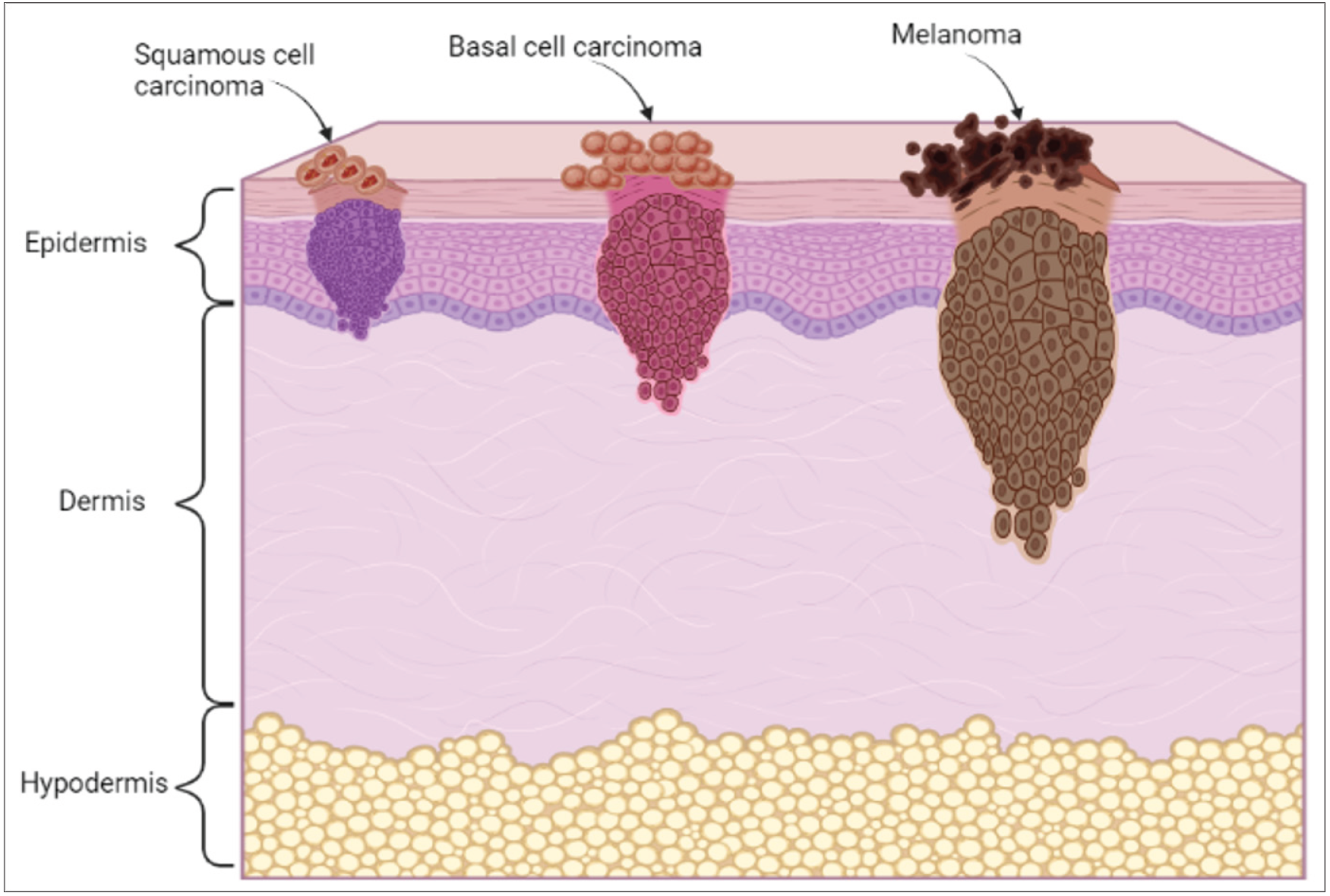
- Types of skin cancer
Incidence, burden and mortality
According to the global cancer statistics (GLOBOCAN) 2020, the estimates of worldwide incidence and mortality of 36 cancer types in 185 countries were analysed, and it was observed that skin cancer accounted for 1,198,073 new cases every year, which represents 6.2% of the total combined cancer cases in 2020 (Figure 3a).2 The number of deaths due to skin cancer globally was estimated to be 63,731, accounting for 0.6% of total deaths reported in the year 2020.2 It was also observed that melanoma of the skin was the fifteenth most common cause of cancer in males, with an incidence rate of 5.2 per 100,000 males and 0.4 per 100,000 males in countries with higher and lower human development index (HDI), respectively. Figure 3d represents the percentage incidences of melanoma and non-melanoma skin cancers with respect to other cancers in India. However, the mortality rates were least when compared with other cancers, that is, 0.9 per 100,000 males and 0.2 per 100,000 males in higher and lower human development index countries, respectively.2 In the case of females, the incidence rates were approximately two folds lower than the males (i.e. 7.9% in females and 15.1% in males respectively).2
The National Cancer Registry Program and GLOBOCAN 2018 reports were collected and analysed to understand the incidence of skin cancer in India. It is believed that, in the Indian population, the incidence rate of different skin cancers is lower, which is attributed to the protective effects of melanin.6 However, the unavailability of reports from national surveys and cross-country data and indirect indications from several small reports indicate an increase in the incidences of skin cancer in the country. The incidence of non-melanoma skin cancers and melanomas in India, specifically among the male population, was observed to be the highest in the north-eastern region. Additionally, among males, the incidence of non-melanoma skin cancers and melanoma was observed highest in Pasighat and Nagaland, respectively. However, melanomas and non-melanoma skin cancers were highest among females in the Northern (Delhi) and north-eastern regions (Pasighat). Amongst melanoma and non-melanoma skin cancers, the latter was found to be more common among the Asian population.7 Figure 3b represents the incidence of non-melanoma skin cancers worldwide in both sexes. It can be seen that the incidence in Asian countries is 7.26%. However, the mortality rate due to all cancers is relatively high in Asian countries, that is, 43.5%, as shown in Figure 3c.8
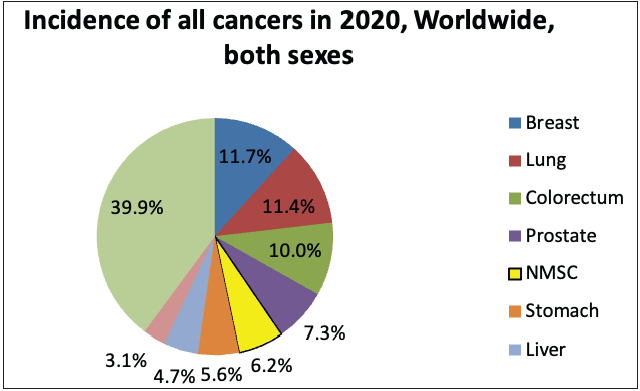
- Pie charts representing status of skin cancers in India and worldwide
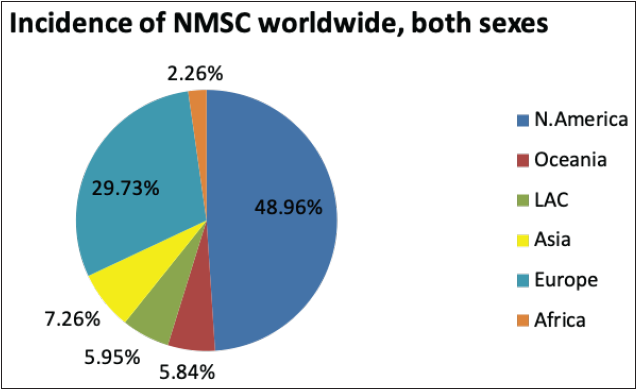
- Pie charts representing status of skin cancers in India and worldwide
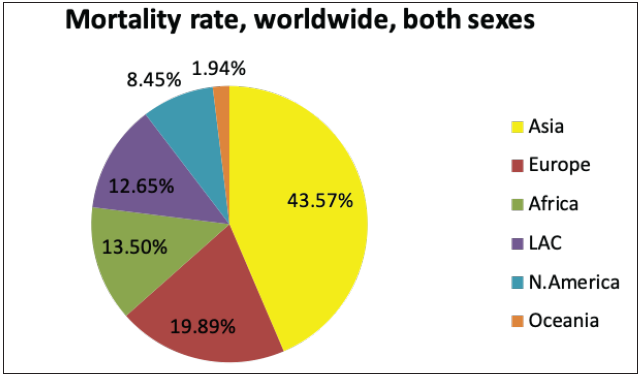
- Pie charts representing status of skin cancers in India and worldwide
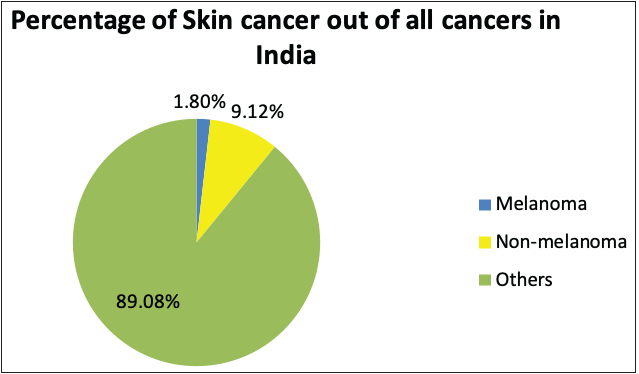
- Pie charts representing status of skin cancers in India and worldwide
Causes and risk factors
Skin cancer is caused due to mutations in the DNA of the skin cells, which leads to uncontrolled growth and formation of tumours. However, the combination of environmental and genetic factors is responsible for the risk of developing skin cancer. Long-term exposure to ultraviolet rays remains one of the most common causes. This chronic ultraviolet exposure has been associated with 90% of cutaneous squamous cell carcinoma and basal cell carcinoma cases and 65% of cutaneous melanomas.9
Current treatment strategies
The gold standard strategy for diagnosing non-melanoma skin cancers is primary physical examination, followed by biopsy and histopathologic examination.10 However, squamous cell carcinoma is susceptible to metastasize to adjacent tissues and therefore a thorough lymph node examination is also required for a complete diagnosis. To avoid invasive procedures for diagnostic purposes, several non-invasive tools have been developed, including dermoscopy, multiphoton microscopy, confocal microscopy, Raman spectroscopy, cross-polarized light and fluorescence photography and coherence tomography with high-frequency ultrasound.10 These technologies help in characterising the features of non-melanoma skin cancers before going with the biopsy method. Dermoscopy, which is mainly used to identify melanocytic lesions, also screens squamous cell carcinoma and basal cell carcinomas.11,12 The most commonly used technique for treating non-melanoma skin cancers is the surgical removal of the lesion, which has the highest curing rate among other existing methods.13,14 Other methods which are employed to treat cases of non-melanoma skin cancers include cryotherapy, laser therapy, pharmacotherapy, radiation therapy and photodynamic therapy (PDT).15,16 However, a detailed discussion about the current treatment or management strategies is out of scope of this review.
Vitamin D
Introduction
Vitamin D, also known as calciferol, belongs to the family of fat-soluble vitamins that promote calcium absorption, regulate bone mineral metabolism and maintain muscle function.17 In nature, vitamin D exists in two primary forms, that is, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) derived from animal or plant sources. Because the human body is not able to synthesise vitamin D2, it is generally added to foods. In contrast, vitamin D3 is synthesised in the skin of the human body from endogenous 7-dehydrocholesterol through photochemical reaction by ultraviolet radiation. However, it can also be taken via the consumption of specific foods.18 Moreover, because vitamin D3 is pharmacologically more potent than the D2 form, it is generally preferred as a treatment option for vitamin D deficiency.19 However, vitamin D3 is frequently used for the treatment of medical conditions such as refractory rickets and hypothyroidism. Apart from medical uses, it is also commonly used to supplement individuals suffering from low vitamin D levels in their bodies.
Synthesis and metabolism in the human body
The endogenous synthesis of vitamin D3 begins with the skin exposure to ultraviolet-B radiation of wavelength between 295 nm and 315 nm.20 Upon exposure to ultraviolet-B, the provitamin D3 (7-dehydrocholesterol), a compound present in skin, is photo-isomerised into previtamin D3 (precholecalciferol), which is converted into vitamin D3 via a heat-dependent reaction.21,22 Later, the hydroxylation of vitamin D3 occurs in the liver, which uses 25-hydroxylase to convert vitamin D3 to 25-hydroxy-vitamin D3 (25(OH)D), also known as calcidiol. The calcidiol is further hydroxylated in the kidneys to form calcitriol, an active metabolite in the hormonal form, with the help of the 1-α-hydroxylase enzyme. The flow chart of vitamin D synthesis and its metabolism is shown in Figure 4.23 The figure depicts the endogenous synthesis and metabolism pathway in the human body. To quantify vitamin D levels in the body, serum-free calcidiol is the best candidate because it has a half-life of 2–3 weeks, and it can be easily measured as well.24,25
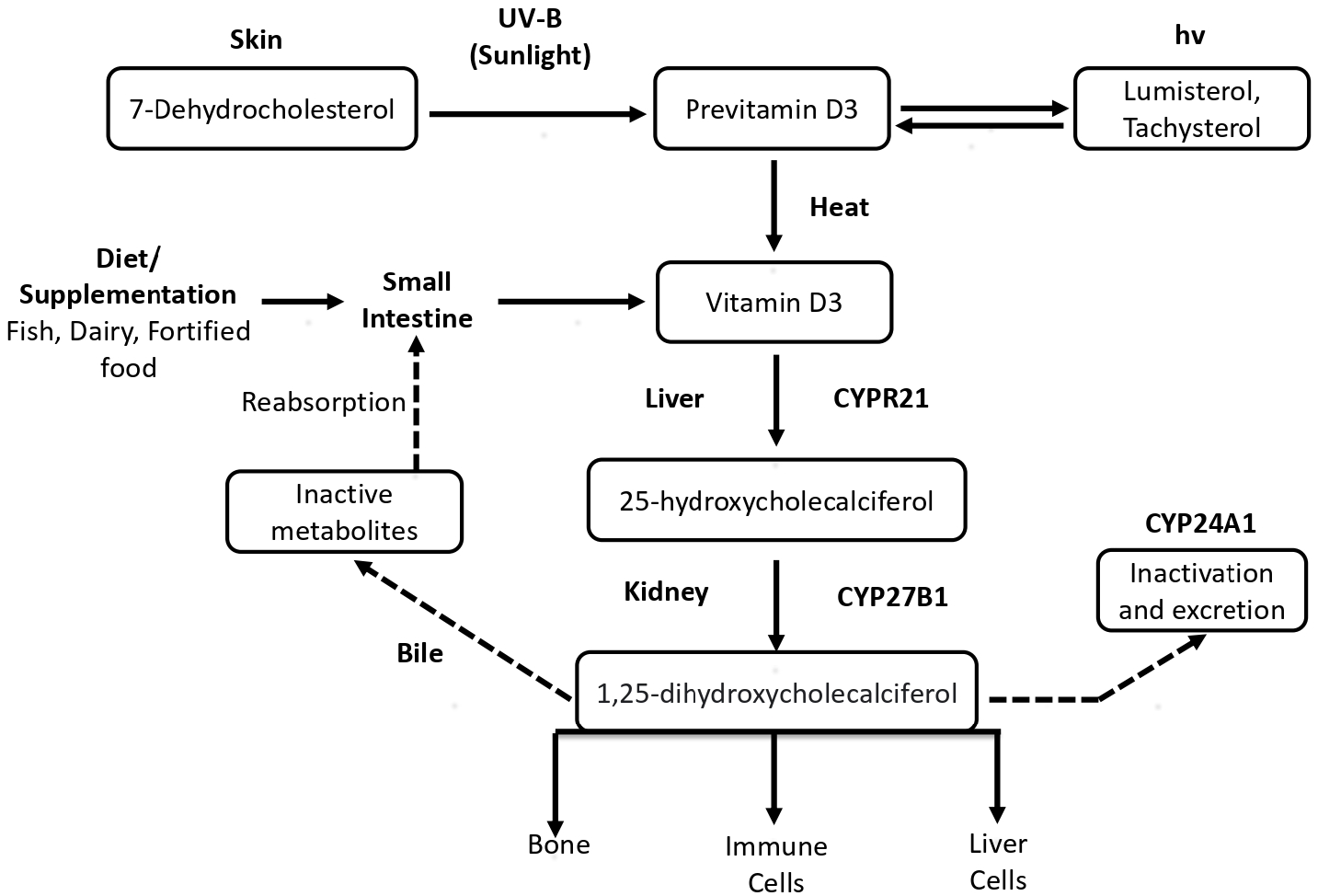
- Endogenous synthesis of vitamin D and its metabolism
Source
Vitamin D can be obtained via three resources, namely, via conversion of 7-dehydrocholesterol into vitamin D3 (cholecalciferol) upon sun exposure, from food and supplements. Dietary sources include natural food such as fish, beef liver, egg yolk, cheese and mushrooms. The Food and Drug Administration (FDA) has also approved ultraviolet-treated mushroom powders as a source of vitamin D2 and as a food additive.26 Table 1 shows the different food sources of vitamin D.27 Sun exposure is one of the prominent sources of vitamin D, which fulfills its daily requirement. Specifically, ultraviolet-B, which falls in the wavelength range of 290–320 nm, is required to synthesise vitamin D3. This radiation has poor penetration through glass, and therefore, the sun’s exposure indoors through a window does not help in vitamin D synthesis.28 According to the literature, approximately 5–30 mins of sun exposure, twice a week or daily without sunscreen, is crucial for the synthesis of vitamin D that is adequate for the body.28,29 Various other factors such as latitude, color and season also affect vitamin D synthesis in the body.29 However, it is important to note that its overall synthesis can occur within the epidermis in order to associate this potentially with skin cancer.30 Induction of 25-hydroxylase mRNA release by vitamin D3 and ultraviolet-B radiation leads to the activation of 1,25(OH)2D that causes local effects within the cell.31 Within the epidermis, the 2D action takes place via vitamin D receptor (VDR), which helps in the regulation of proliferation in the basal cells and also promotes the differentiation of keratinocytes as they start to form the upper layer of the epidermis.30,32
| Food source | UI per serving |
|---|---|
| Cod liver oil (1 tablespoon/5 mL) | 1360 |
| Swordfish (3 ounces) | 566 |
| Sardines in oil (3 ounces) | 450 |
| Salmon (3 ounces) | 447 |
| Mackerel (3 ounces) | 30 |
| Tuna in oil (3 ounces) | 200 |
| Tuna fish 3 ounces | 154 |
| Orange juice fortified with vitamin D, 1 cup (check product labels, as amount of added vitamin D varies) | 137 |
| Milk, non-fat, reduced fat, and whole, vitamin D-fortified, 1 cup | 115–124 |
| Yogurt, fortified with 20% of the DV for vitamin D, 6 ounces (more heavily fortified yogurts provide more of the DV) | 80 |
| Liver, beef, cooked, 3 ounces | 42 |
| Egg, 1 large (vitamin D is found in yolk) | 41 |
| Ready-to-eat cereal, fortified with 10% of the DV for vitamin D, 0.75–1 cup (more heavily fortified cereals might provide more of the DV) | 40 |
| Cheese, Swiss, 1 ounce | 6 |
| Mushrooms, morel, raw (½ cup) | 40 |
DV: Daily value
Vitamin D and Skin Carcinogenesis
It is crucial and challenging to identify the role of vitamin D in the etiology of skin cancer in humans as exposure to sun is required for vitamin D production which may also, in some instance, is responsible for skin cancer. Various studies have been generated from in vitro and in vivo trials along with epidemiological and genetic studies in humans for deciphering the relationship between sun exposure, vitamin D and skin cancer.33 Some include genetic studies on normal and cancerous skin cells, suggesting the role of vitamin D in specific vitamin D pathway gene abnormalities. Though some clinical data provide information linking the incidence or progression of skin cancer with vitamin D levels, it becomes complicated to differentiate the effects of vitamin D versus the effects of sun exposure as the latter is a principal determinant of vitamin D levels.33
Shreds of evidence suggest that endogenous synthesis of vitamin D, metabolism and vitamin D receptor–regulated transcripts can control the growth and behaviour of cancer cells.34 Several clinical trials and in vitro studies have demonstrated the inhibitory effects of vitamin D in non-melanoma skin cancer, and their positive effects in the prevention of skin cancer. However, the exact relationship between these metabolic enzymes (CYP27A1, CYP27B1 and CYP24A1) and vitamin D receptor in the genesis and progression of non-melanoma skin cancer is not fully understood.35
The enzyme CYP27A1 is a mitochondrial protein which plays an important role in the overall cholesterol homeostasis. The enzyme CYP27B1 is located in inner mitochondrial membrane and is involved in hydroxylation of 25(OH)D at the 1-alpha position. The enzyme CYP24A1 regulates the levels of vitamin D3 by initiating the degradation of 1,25(OH)2D3. In a study of aggressive type melanomas by Brożyna et al.,36 a decrease in the enzymatic activity of one of the above enzymes, CYP27B1, during melanoma progression was also reported.36
Nemazannikova et al.35 studied the intra tumoral expression of mitochondrial vitamin D metabolic enzymes and vitamin D receptors in non-melanoma skin cancer using standard immunohistochemistry. In the case of basal and squamous cell carcinomas, there was a strong expression of CYP27A1 that was associated with the CYP24A1 expression. Moreover, the above association was observed more significantly in case of squamous cell carcinoma. However, in the case of association between CYP27B1 and CYP24A1 expression levels, an inverse significant association is observed in all non-melanoma skin cancer cases.35 Ellison et al.37 observed an enhanced susceptibility to ultraviolet-induced tumorigenesis in murine skin after the inactivation of vitamin D receptors. They studied the role of vitamin D receptors in skin cancer. They observed a rapid development of chemically induced skin tumours in vitamin D receptor (−/−) mice; however, there was no such observation in the case of CYP27B1 (−/−) and wild-type mice [vitamin D receptor (+/+)]. This indicated that it is a vitamin D receptor and not a vitamin D3 ligand, which is crucial for the protective effects against skin tumorigenesis. Moreover, there was a rapid development of ultraviolet-induced tumours in the case of vitamin D receptors (−/−) mice with greater penetrance than in wild-type mice. Also, the levels of p53 proteins were observed to be upregulated at similar rates in ultraviolet-treated keratinocytes of vitamin D receptor (−/−) than wild-type mice. Additionally, the results from in-vivo experiments reported an increased development of ultraviolet-induced tumours with higher penetrance when compared with wild-type mice, suggesting a critical role of vitamin D receptor in the repair and removal of severely damaged keratinocytes and adaptation of the skin to chronic ultraviolet exposure.37
Muralidhar et al.38 demonstrated the relationship between vitamin D and vitamin D receptor signaling using 703 primary melanoma transcriptomes.38 Additionally, they tried to replicate these findings in The Cancer Genome Atlas metastases. This study demonstrated the protective effects of vitamin D–vitamin D receptor signaling for melanoma-related deaths via inhibiting the Wnt/β-Catenin pathway, impacting melanoma progression and antitumour immune response. Moreover, the overexpression of vitamin D receptor in murine melanoma cells resulted in fewer pulmonary metastases than the controls in tail vein metastasis assays. Therefore, the expression profile of vitamin D receptor could be used as a potential biomarker to differentiate melanoma patients that might respond better to immunotherapy.37
On the other hand, Burns et al. 39 studied the association of vitamin D receptor polymorphisms with the risk of non-melanoma skin cancer in adults. The importance of vitamin D signaling confers protective effects against non-melanoma skin cancer upon ultraviolet-B exposure, which is mediated via vitamin D receptor.39 However, recent examination of single nucleotide polymorphisms (SNPs) in the vitamin D receptor has resulted in contradictory results, which causes ambiguity, whether these polymorphisms increase an individual’s risk of developing non-melanoma skin cancer. Hence, they examined the association of vitamin D receptor polymorphisms with non-melanoma skin cancer development and participants’ demographic characteristics.
Basal cell carcinomas also arise due to defects in another major signaling pathway known as the Hedgehog pathway.40 Sonic hedgehog (Shh) instigates the nuclear activation transcription factor Gli, which increases the anti-apoptotic and cyclin factors expressions with simultaneous suppression in keratinocyte differentiation genes.41 The components of the sonic hedgehog pathway get upregulated upon chemical carcinogenesis and ultraviolet-B exposure to the epidermal cells.42 According to a study, in vitamin D receptor–null mice, sonic hedgehog is elevated in the epidermis, utricles of dysplastic hair follicles and the outer rim of cells that make up the lipid-laden cells in dermal cysts.39 These findings propose significant antagonistic functions of vitamin D receptor towards the sonic hedgehog signaling pathway and play a protective role during basal cell carcinoma formation.
Moreover, it is also suggested that vitamin D plays a significant regulatory role in the formation and prognosis of cutaneous malignant melanoma. Recently reported genetic variations in the vitamin D receptor gene are potent risk factors for the development and pathogenesis of melanoma, studied via single nucleotide polymorphism.43 However, the causal effect relationship is unknown; it was observed that the individuals with low levels of 25(OH)D showed increase in cutaneous malignant melanoma.44 In vitro studies also suggested the anti-proliferative effects of vitamin D on cultured melanoma cells as vitamin D blocks the proliferation of melanoma cells.45 Some recent studies also demonstrated that responsive (MeWo), and resistant (SK-Mel-5) melanoma cell lines are implicated in the anti-proliferative effects of vitamin D governed by the degree by which vitamin D receptor and Cyp24a1 is induced.46 However, it was concluded that most melanoma cell lines were resistant to the anti-proliferative effects of vitamin D3 and that vitamin D would positively affect a minority of melanoma cases in patients. On the other hand, co-treatment of melanoma cell lines with drugs that modulate epigenetics increased the responsiveness to vitamin D3, suggesting possible combined therapy in some instances.47
Protective effects of vitamin D from ultraviolet rays
The long wave ultraviolet-A and short wave ultraviolet-B are the types of ultraviolet rays in sunlight that damage DNA. In the case of ultraviolet-A, it penetrates into the deeper layers of skin and leads to production of free radicals, which then cause oxidative and DNA damage. Whereas ultraviolet-B usually affects the superficial layers of the skin, causing DNA damage leading to driver and bystander mutations followed by cellular transformations and malignancies.48 Despite the photocarcinogenic49 and immunosuppressive effects50 of ultraviolet-B components, it is also crucial for the synthesis of vitamin D for its various functions in the body. In order to repair DNA damage post-ultraviolet exposure, the growth of the cell is arrested, wherein faulty repair or improper removal of damaged cells results in clonally expanded cells that constitute tumours.37,51–54 In vivo studies, trying to understand the role of vitamin D receptors upon short-term ultraviolet exposure, observed a decrease in the proliferation of normal mouse keratinocytes, however, the vitamin D receptor–ablated cells showed more proliferation. These results suggest the role of vitamin D receptor in promoting growth arrest upon short ultraviolet treatment.37 Additionally, fewer apoptotic cells were observed in vitamin D receptor–null skin than in normal skin post-ultraviolet exposure, demonstrating the critical role of vitamin D receptor in eliminating severely damaged cells. Apart from ultraviolet exposure, the application of either oral or topical agents containing carcinogens also increases skin tumours in vitamin D receptor–null mice.54
Dixon et al.55 studied the inhibitory effects of 1α,25(OH)-vitamin D, and non-genomic vitamin D analogue against ultraviolet-induced skin carcinogenesis.55 They demonstrated that both vitamin D3 and 1α,25(OH)₂-lumisterol, a conformational analogue, were effective inhibitors of ultraviolet damage in an immunocompetent mouse (Skh:hr1) model susceptible to ultraviolet-induced tumours. Additionally, in mice, they observed inhibition of the development of skin cancer in the case of squamous cell carcinoma. These observations suggest the protective role of these compounds in the prevention of skin carcinogenesis.
In another study by Zhang et al.,53 they investigated the role of DNA damage-inducible transcript 4 (DDIT4) in human squamous cell carcinoma.52 They observed a significant suppression of DDIT4 in the case of squamous cell carcinoma and A431 cell lines. Moreover, the reduction of DDIT4 accelerated the proliferation of keratinocytes but obstructed the autophagy flux through the mTORC1 pathway by affecting the downstream S6 kinase1, 4E-BP1, beclin1 and LC3 II/I. The in vivo studies revealed the anti-carcinogenic effect of vitamin D in case of xenograft tumor–bearing mice treated with different concentrations of 1,25(OH)2D3 and DDIT4.
Apart from environmental factors, genetic factors also play a role in the development of skin cancer. For example, people with fairer skin are at an increased risk of developing skin cancer. Moreover, individuals with freckling and red hair carry two copies of the R allele variant of the MC1R gene and are also at increased risk of developing skin cancer. Xeroderma pigmentosum, a rare autosomal recessive disease, is a condition where the individuals suffering from this disease cannot repair the ultraviolet-induced damage leading to mutations in the genes that form an integral part of the nucleotide excision repair pathway. This pathway plays a vital role in repairing the damage caused due to ultraviolet rays.18 This abnormal condition predisposes an individual to an increased risk of developing freckling, sunburn and early childhood malignancies.
Daily requirement of vitamin D and preventive strategies
The daily requirement of vitamin D is dependent on an individual’s age, but the standard concentration required for daily intake has been a topic of discussion. The Institute of Medicine, an independent, non-profit organisation, has provided data on daily vitamin D intake for individuals of different age groups, as shown in Table 2.18 The daily intake of vitamin D varies with age of an individual. For example, infants require a daily intake of 400 IU vitamin D3, whereas individuals in the age group 1–70 years require 600 IU and for individuals above 70 years of age, the recommended intake is 800 IU/day.
| Age group (years) | Daily intake (IU/d) |
|---|---|
| <1 | 400 |
| 1–70 | 600 |
| >70 | 800 |
IU: International unit; d: Day
Seamans et al.56 validated the reliability and robustness of circulating levels of vitamin D as a marker of vitamin D status in the human body.56 According to the American Endocrine Society, serum levels of vitamin D under 20 ng/mL are considered deficient. In contrast, serum levels in the range of 20–30 ng/mL are considered insufficient and levels above 30 ng/mL are considered essential for maintaining good health.57
The Institute of Medicine has specified 20 ng/mL as the lower limit indicating healthy and sufficient vitamin D levels [Table 3].18 A higher level of vitamin D in serum, particularly above 200 ng/mL, is considered toxic, and the associated symptoms include weight loss, polyuria, anorexia and cardiac arrhythmia. Moreover, vitamin D toxicity also increases the risks of calcification of tissues and blood vessels and formation of kidney stones.27
| nmol/L | ng/mL | Health status |
|---|---|---|
| <30 | <12 | Linked with deficiency of vitamin D, which can cause rickets and osteomalacia in infants and adults, respectively |
| 30–50 | 12–20 | Considered as inadequate for bone health |
| ≥50 | ≥20 | Considered as adequate for bone and general health |
| >125 | >50 | High levels can be linked to potential adverse effects (>60 ng/mL) |
A few lifestyle changes in daily life could help maintain healthy levels of vitamin D in the body and prevent its deficiency. This includes getting enough sun exposure as it is the primary source for vitamin D synthesis, daily intake of foods rich in vitamin D and being proactive to maintain overall health. However, for patients who have skin cancer and cannot get sensible sun exposure, the administration of vitamin D as a supplement could be employed as an alternative way to meet the daily requirements.
Effects of vitamin D on tumor microenvironment
Studies also demonstrated that vitamin D inhibits the growth of cancer cells and cancer stem cells58 and plays an essential role in regulating the tumor microenvironment. Various studies suggest regulating tumor initiation and metastasis by influencing cell-microenvironment interactions by vitamin D.59 A large number of tumor cells and a small portion of cancer stem cells exist in the stromal microenvironment, along with vasculature for nourishment, cancer-associated fibroblasts, immune cells and extracellular components. It is well demonstrated that chronic inflammation is the main contributor to the initiation of tumours.60 In this regard, vitamin D is known to exert its anti-inflammatory effects with tumor-specific outcomes. For example, vitamin D3 is responsible for the inhibition of the prostaglandin pathway that is involved in pro-inflammatory responses via inhibition of cyclooxygenase-2, prostaglandin receptors and degradation of prostaglandin in prostate61,62 and breast63 tumor cells.
Vitamin D3 is also known to suppress the p38 MAPK-regulated pro-inflammatory pathway in prostate cancer cells.64 Blocking of pro-inflammatory nuclear factor kappa B (NFκB) pathway was also reported via vitamin D3, and also the inhibition of Akt within macrophages.65 Modulation of cancer and immune cell phenotypes by suppressing pro-inflammatory cytokines such as TNF-α and IL-6 by vitamin D3 was also demonstrated.66 Vitamin D receptor–null mice developed impaired cutaneous wound healing at the expense of F4/80+ macrophages recruited to the wound site.67 Whether this impaired innate immune response observed in the skin can also contribute to the tumor microenvironment remains unexplored.
Most important question
Vitamin D: How much is enough?
Various studies cited above correlated elevated serum levels of vitamin D with higher exposure to the sun. A study by Hart et al.68 demonstrated possible independent effects of sun exposure on health, particularly immune-mediated effects not occurring through a vitamin D pathway.68 This can be related to an issue linked to skin cancer development and its prognosis. Berwick et al.69 were the first to validate the advantageous effect of the sun’s ultraviolet rays on melanoma prognosis. The study reported that high intermittent exposure to the sun is associated with low death risk in patients with melanoma within 5 years of diagnosis.69
Arguments have always been there regarding the optimal levels of vitamin D required for good health conditions. Various observational studies associate low levels of vitamin D with an increased risk of internal cancers. However, considering the various reviews of associations with a wide range of disease outcomes, the United States Institute of Medicine suggested a significant causal association of vitamin D levels with good bone health, and recommended serum levels of 50 nmol/L should be considered sufficient.18
The ultraviolet index, or UVI, is an international standard measurement of the strength of sunburn-producing ultraviolet radiation at a particular place and time. There was a consensus that vitamin D levels less than 25 nmol/L are considered deficient.70 The Institute of Medicine concluded that vitamin D levels less than 30 nmol/L should be considered a deficiency risk.18 Upon diagnosis of skin cancer or in persons with increased risk of skin cancer, it may be appropriate to avoid sun exposure as much as possible and ensure adequate vitamin D status. There is considerable evidence that suggests a fall in vitamin D status following a diagnosis of skin cancer, for example the study by Idorn et al.71 The findings from this study suggested that patients with cutaneous malignant melanoma had a higher ultraviolet exposure in the summer before the diagnosis than did controls.
The production of vitamin D is primarily induced only by ultraviolet-B radiation. It is thus most effective when the intensity of ultraviolet-B is the highest throughout the day, that is, at noon, with simultaneous minimization of the risk of erythema.72 A general notion that directly correlates the production of vitamin D3 with the surface area of the skin is that the more the skin area, the more will be the production of vitamin D3.
On the other hand, erythema of skin or epidermal cells induces DNA damage and increases the risk of skin cancer. As a result, there should be maximum exposure to the skin when ultraviolet index is highest but for a short period for adequate vitamin D production.
Although, the absolute difference in time required to achieve the above condition when vitamin D3 production and erythema being optimal at high ultraviolet index is narrow. For example, based on current dose-response models and International Commission on Illumination (CIE) action spectra, when the ultraviolet index is 12, it takes less than 5 min to produce an acceptable daily dose of vitamin D with only face and arms exposed. On the other hand, it takes about 15 min for somebody to experience erythema.73 While extreme caution would be required to avoid harmful levels of sun exposure, the appropriate strategy for people at high risk of skin cancer may be to apply sunscreen to the hands, face and arms routinely but to deliberately expose less frequently exposed areas of skin for a short time at a high ultraviolet index.
Future Perspective
The pleiotropic activities of vitamin D, in addition to the regulation of body calcium homeostasis, and anti-carcinogenic activities are consistent with the actions of multiple vitamin D derivatives produced in the human body. In vivo and in vitro studies were reviewed to document the role of vitamin D in photoprotection and the prevention or attenuation of non-melanoma skin cancers. Although the role of vitamin D in reducing the risk of skin cancer has been well established, its mechanism of action is not well understood. More experimental data on the mechanism of vitamin D in reducing the risk of skin cancer is required, which may open up novel opportunities for its diagnosis and treatment. Also, not all the tumor-suppressor actions of vitamin D receptor appear to be ligand-mediated, and there are a few studies where the vitamin D receptor can function in the absence of a ligand. However, such studies are limited and require more elaborative work.
Conclusion
The synthesis of vitamin D via the vitamin D pathway plays a significant role in maintaining the levels of circulating vitamin D in the body. Also, the same pathway is crucial for the pathogenesis and the progression of cutaneous melanoma, illustrating the gene–environment interactions. However, to understand these intricate mechanisms linking vitamin D and skin cancer, there is an increased need for well-designed prospective studies that will include information about genotypes and phenotypes of vitamin D metabolism and open up novel opportunities to establish a possible link between vitamin D and skin cancer. In summary, recent advances in vitamin D, skin biology and pharmacology are providing new and exciting opportunities in skin healthcare for treating different pathologies.
Acknowledgement
The authors would like to thank Dr. Utpreksha Vaish and Dr. Monika Chauhan for their valuable suggestions in the completion of this manuscript.
Declaration of Patient Consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- A brief history of cancer: Age-old milestones underlying our current knowledge database: A brief history of cancer. Int J Cancer. 2015;136:2022-36.
- [CrossRef] [PubMed] [Google Scholar]
- Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: C.A. Cancer J Clin. 2021;71:209-49.
- [Google Scholar]
- Screening for skin cancer: An update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;150:194-98.
- [CrossRef] [PubMed] [Google Scholar]
- NCI Dictionary of Cancer Terms. National Cancer Institute 2011-02-02. Retrieved 9 November 2016.
- Incidence of melanoma and non-melanoma skin cancers in Indian and the global regions. J Cancer Res Ther. 2021;17(4):906-11.
- [Google Scholar]
- Nonmelanoma skin cancer in India: Current scenario. Indian J Dermatol. 2010;55:373.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Globocan report 2020. Available from: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf
- Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis. 2014;1:188-98.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dermoscopy, confocal microscopy and other non-invasive tools for the diagnosis of non-melanoma skin cancers and other skin conditions. Acta Derm Venereol. 2017;Suppl 218:22-30.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy and its impact on skin cancer diagnostics. Drugs Dermatol. 2010;9:129-30.
- [Google Scholar]
- Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48.
- [CrossRef] [PubMed] [Google Scholar]
- Multiprofessional guidelines for the management of the patient with primary cutaneous squamous cell carcinoma. Br J Dermatol. 2002;146:18-25.
- [CrossRef] [PubMed] [Google Scholar]
- Photodynamic therapy and non-melanoma skin cancer. Cancers (Basel). 2016;8:98.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Radiation therapy in dermatology: Non-melanoma skin cancer. J Drugs Dermatol. 2017;16:464-69.
- [PubMed] [Google Scholar]
- Dietary reference intakes for calcium and vitamin D. Washington, D.C.: National Academies Press; 2011.
- Vitamin D3 is more potent than vitamin D2 in humans. J Clin Endocrinol Metab. 2011;96:E447-52.
- [CrossRef] [PubMed] [Google Scholar]
- A revised action spectrum for vitamin D synthesis by suberythemal UV radiation exposure in humans in vivo. Proc Natl Acad Sci. 2021;118:e2015867118.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evaluation of an ultraviolet B light emitting diode (LED) for producing vitamin D3 in human skin. Anticancer Res. 2020;40:719-22.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D and the liver—Correlation or cause? Nutrients. 2018;10:496.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Regulation of 25-hydroxyvitamin D-1-hydroxylase and 24-hydroxylase in keratinocytes by PTH and FGF23. Exp Dermatol. 2018;27:1201-09.
- [CrossRef] [PubMed] [Google Scholar]
- UV increases skin-derived 1α,25-dihydroxyvitamin D3 production, leading to MMP-1 expression by altering the balance of vitamin D and cholesterol synthesis from 7-dehydrocholesterol. J Steroid Biochem Mol Biol. 2019;195:105449.
- [CrossRef] [PubMed] [Google Scholar]
- Food additives permitted for direct addition to food for human consumption; vitamin D2 mushroom powder. Federal Register. 2020;85:41916-20.
- [Google Scholar]
- Vitamina D y la piel. Una revisión para dermatólogos. Actas Dermosifiliogr. 2019;110:262-72.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D for health: A global perspective. Mayo Clin Proc. 2013;88:720-55.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466-79.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D and the skin: Physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3-19.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Expression of vitamin D3 25-hydroxylase (CYP27) mRNA after induction by vitamin D3 or UVB radiation in keratinocytes of human skin equivalents—A preliminary study. Arch Derm Res. 1999;291:507-10.
- [CrossRef] [PubMed] [Google Scholar]
- 1,25-dihydroxyvitamin D3 and malignant melanoma: The presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083-86.
- [CrossRef] [PubMed] [Google Scholar]
- Skin cancer and vitamin D: An update. Melanoma Manag. 2015;2:51-61.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Analysis of 1,25-dihydroxyvitamin D3 receptors (VDR. In basal cell carcinomas. Am J Pathol. 1999;155:583-89.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Vitamin D enzymes (CYP27A1, CYP27B1, and CYP24A1) and receptor expression in non-melanoma skin cancer. Acta Biochim Biophys Sin (Shanghai). 2019;51:444-47.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of the vitamin D-activating enzyme 1α-hydroxylase (CYP27B1) decreases during melanoma progression. Hum Pathol. 2013;44:374-87.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol. 2008;128:2508-17.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Vitamin D-VDR signalling inhibits Wnt/β-catenin-mediated melanoma progression and promotes antitumor immunity. Cancer Res. 2019;79:5986-98.
- [CrossRef] [PubMed] [Google Scholar]
- Association of vitamin D receptor polymorphisms with the risk of non-melanoma skin cancer in adults. JAMA Dermatol. 2017;153:983-89.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The vitamin D receptor is required for activation of cWnt and hedgehog signalling in keratinocytes. Mol Endocrinol. 2014;28:1698-706.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet. 2000;24:216-17.
- [CrossRef] [PubMed] [Google Scholar]
- Overexpression of hedgehog signalling is associated with epidermal tumor formation in vitamin D receptor-null mice. J Invest Dermatol. 2011;131:2289-97.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Vitamin D receptor polymorphisms and melanoma. Oncol Lett. 2019;17:4162-69.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Vitamin D status and risk for malignant cutaneous melanoma: Recent advances. Eur J Cancer Prev. 2017;26:532-41.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Differential apoptotic response of human melanoma cells to 1 alpha,25-dihydroxyvitamin D3 and its analogues. Cell Death Differ. 1998;5:946-52.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro comparison of the vitamin D endocrine system in 1,25(OH)2D3-responsive and -resistant melanoma cells. Cancer Biol Ther. 2007;6:48-55.
- [CrossRef] [PubMed] [Google Scholar]
- Signature of VDR miRNAs and epigenetic modulation of vitamin D signalling in melanoma cell lines. Anticancer Res. 2012;32:383-89.
- [PubMed] [Google Scholar]
- Ultraviolet radiation exposure and its impact on skin cancer risk. Semin Oncol Nurs. 2016;32:241-54.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The vitamin D questions: How much do you need and how should you get it? J Am Acad Dermatol. 2006;54:301-17.
- [CrossRef] [PubMed] [Google Scholar]
- Ultraviolet radiation and immunosuppression. Br J Dermatol. 2009;161 Suppl 3:90-95.
- [CrossRef] [PubMed] [Google Scholar]
- Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Res. 2016;36:1345-56.
- [PubMed] [Google Scholar]
- Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J Invest Dermatol. 2007;127:707-15.
- [CrossRef] [PubMed] [Google Scholar]
- DNA damage-inducible transcript 4 is an innate guardian for human squamous cell carcinoma and a molecular vector for anti-carcinoma effect of 1,25(OH)2 D3. Exp Dermatol. 2019;28:45-52.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726-76.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- 1α,25(OH)₂-vitamin D and a nongenomic vitamin D analogue inhibit ultraviolet radiation-induced skin carcinogenesis. Cancer Prev Res (Phila). 2011;4:1485-94.
- [CrossRef] [PubMed] [Google Scholar]
- Existing and potentially novel functional markers of vitamin D status: A systematic review. Am J Clin Nutr. 2009;89:1997S-2008S.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-30.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting cancer stem cells in solid tumours by vitamin D. J Steroid Biochem Mol Biol. 2015;148:79-85.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Vitamin D in cancer chemoprevention. Pharm Biol. 2015;53:1399-434.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular pathways mediating the anti-inflammatory effects of calcitriol: Implications for prostate cancer chemoprevention and treatment. Endocr Relat Cancer. 2010;17:R19-38.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005;65:7917-25.
- [CrossRef] [PubMed] [Google Scholar]
- 1,25-Dihydroxyvitamin D3 inhibits growth of the breast cancer cell line MCF-7 and downregulates cytochrome P4501B1 through the COX-2/PGE2 pathway. Oncol Rep. 2012;28:2131-37.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibition of p38 by vitamin D reduces interleukin-6 production in normal prostate cells via mitogen-activated protein kinase phosphatase 5: Implications for prostate cancer prevention by vitamin D. Cancer Res. 2006;66:4516-24.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J Biol Chem. 2014;289:11681-94.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- 1α,25-Dihydroxyvitamin D3 modulates the interaction between immune and colon cancer cells. Biomed Pharmacother. 2012;66:428-32.
- [CrossRef] [PubMed] [Google Scholar]
- Ligand-dependent actions of the vitamin D receptor are required for activation of TGF-β signalling during the inflammatory response to cutaneous injury. Endocrinology. 2013;154:16-24.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Modulation of the immune system by UV radiation: More than just the effects of vitamin D? Nat Rev Immunol. 2011;11:584-96.
- [CrossRef] [PubMed] [Google Scholar]
- Sun exposure and mortality from melanoma. J Natl Cancer Inst. 2005;97:195-99.
- [CrossRef] [PubMed] [Google Scholar]
- Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18-28.
- [CrossRef] [PubMed] [Google Scholar]
- Sun exposure before and after a diagnosis of cutaneous malignant melanoma: Estimated by developments in serum vitamin D, skin pigmentation and interviews: Sun exposure before and after a diagnosis of CMM. Br J Dermatol. 2011;165:164-70.
- [CrossRef] [PubMed] [Google Scholar]
- Serum 25-hydroxyvitamin-D responses to multiple UV exposures from solaria: Inferences for exposure to sunlight. Photochem Photobiol Sci. 2012;11:1174.
- [CrossRef] [PubMed] [Google Scholar]
- UV radiation: Balancing risks and benefits. Photochem Photobiol. 2009;85:88-98.
- [CrossRef] [PubMed] [Google Scholar]






