Translate this page into:
Accuracy of serration pattern analysis by direct immunofluorescence in subepidermal autoimmune blistering diseases
Corresponding author: Dr. Debajyoti Chatterjee, Department of Histopathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. devchat1984@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sood R, Chatterjee D, De D, Saikia UN, Mahajan R, Handa S, et al. Accuracy of serration pattern analysis by direct immunofluorescence in subepidermal autoimmune blistering diseases. Indian J Dermatol Venereol Leprol. 2024;90:336-41. doi: 10.25259/IJDVL_20_2023
Abstract
Background
Direct immunofluorescence (DIF) is essential for the diagnosis of sub-epidermal immunobullous diseases (SIBD). Bullous pemphigoid (BP), a sub-epidermal immunobullous disease, shows linear IgG and C3 deposition along the dermo-epidermal junction by DIF. However, similar histological and DIF findings are also seen in epidermolysis bullosa acquisita (EBA). High-power examination of antibody deposition by DIF in a “u” or “n” serrated pattern can help differentiate these two entities.
Aims/Objectives
The aim of this study was to determine the diagnostic accuracy of serration patterns in IgG-mediated sub-epidermal immunobullous disease.
Methods
All cases of IgG-mediated sub-epidermal immunobullous disease diagnosed over the past 2 years and 9 months period and confirmed serologically, were included. Examination of the serration pattern in DIF was assessed on oil emersion. Salt split skin indirect immunofluorescence (SSS IIF), BP180 enzyme-linked immunosorbent assay (ELISA), profile ELISA and BIOCHIP mosaic were performed, wherever available.
Results
This study included 74 cases of bullous pemphigoid, eight cases of mucus membrane pemphigoid (MMP) and one case of epidermolysis bullosa acquisita. The characteristic zigzag “n” pattern was visualised in 66 out of 82 cases (80.5%) of the pemphigoid group (BP + MMP); the single epidermolysis bullosa acquisita case showed the “u” serrated pattern. No statistical correlation was seen between serration pattern and BP180 positivity by ELISA (P = 0.05).
Limitations
The study is limited by the single case of epidermolysis bullosa acquisita (which could be due to rarity of this disease in north Indian population due to genetic variation), lack of detailed serological investigations and immunoblot in all cases.
Conclusion
Serration pattern analysis is an easy-to-interpret and highly useful technique for characterisation of sub-epidermal immunobullous diseases.
Keywords
Autoimmune blistering diseases
bullous pemphigoid
direct immunofluorescence
serration pattern
Plain Language Summary
Sub-epidermal immunobullous diseases have overlapping clinical, histopathological and immunofluorescence features requiring serology based testing by various techniques, few of which are costly and not widely accessible. Moreover, many cases lack circulating antibodies. We sought to determine the diagnostic accuracy of serration patterns in distinguishing between the IgG positive subepidermal immunobullous diseases encountered in our institute over 2 years and detected the zigzag “n” pattern in most pemphigoid patients. Hence, analyzing the biopsies on high power for serration pattern on routinely performed immunofluorescence can be a diagnostic adjunct for pemphigoid group, especially in resource poor settings.
Introduction
Direct immunofluorescence (DIF) is a vital tool in the work up of sub-epidermal immunobullous diseases (SIBD) such as bullous pemphigoid (BP), mucous membrane pemphigoid (MMP), epidermolysis bullosa acquisita (EBA) and linear IgA dermatosis, amongst others. Sub-epidermal immunobullous diseases produce auto-antibodies against basement membrane zone (BMZ) components viz. 180-kDa antigen (BP180), 230-kDa antigen (BP230), laminin 332, collagen VII, laminin γ1(p200), and so on.1 Bullous pemphigoid, one of the most common sub-epidermal immunobullous diseases shows findings similar to epidermolysis bullosa acquisita on histology and direct immunofluorescence [linear IgG and/ or C3 deposition along dermo-epidermal junction (DEJ)]. Precise distinction between them is essential as bullous pemphigoid is treated with topical steroids while epidermolysis bullosa acquisita requires more aggressive management.2 Serological tests such as indirect immunofluorescence (IIF) using salt split skin (SSS), enzyme-linked immunosorbent assay (ELISA), BIOCHIP mosaic, immunoblot and immunoprecipitation can supplement the distinction.3–6 However, ELISA and BIOCHIP are not as widely available as direct immunofluorescence. Hence, examining direct immunofluorescence on high power (60-100X) can be a practical and useful tool in differentiating between various sub-epidermal immunobullous diseases. Two patterns of antibody deposition have been described: “u”- and “n”-serrated; the latter predominates in pemphigoid disorders. The aim of this study was to determine the frequency of serration pattern detection in serologically confirmed IgG-mediated sub-epidermal immunobullous diseases.
Methods
Retrospectively, all IgG mediated sub-epidermal immunobullous diseases cases diagnosed between November 2019 and August 2022 were screened. Two skin biopsies were obtained in each suspected case of the disease, one lesional for histopathological analysis and one perilesional for direct immunofluorescence. After snap freezing at −30°C, tissue was sectioned at 5–6 µm thickness and stained with fluorescein isothiocyanate (FITC)-labelled anti-human IgG, IgA, IgM and C3 antibodies (DAKO). Slides were analysed using fluorescence microscope (Zeiss), and serration pattern was evaluated at 63X and oil immersion (100X) in cases with intact dermo-epidermal junction. In addition, 5 mL blood was collected from patients and the serum was stored at −80°C. Both skin biopsies and the serum were collected from the patient during his or her first visit to the immunobullous disease clinic and before initiation of treatment.
The following investigations were carried out using the sera.
-
Salt split skin indirect immunofluorescence: Performed in 62 patients. The salt split skin sample (in house) was incubated with patient sera (1:10 dilution) for 30 min, followed by washing with phosphate buffer saline (PBS). Then, fluorescein isothiocyanate–labelled anti-human IgG and C3 were added and incubated for 30 min. Roof binding was considered diagnostic for bullous or mucous membrane pemphigoid, while floor binding was suggestive of epidermolysis bullosa acquisita or anti-p200 pemphigoid or anti laminin-332 membrane pemphigoid.
-
BP180 ELISA: Performed in 42 cases with commercially available ELISA kit (Euroimmun, Luebeck, Germany).
-
BIOCHIP mosaic (Six-well plate, Euroimmun, Luebeck, Germany) and/or profile ELISA (Euroimmun, Luebeck, Germany) were carried out in selective cases (n = 13).
Mucous membrane pemphigoid was diagnosed in the presence of predominant mucosal involvement along with intact blisters or erosions irrespective of scarring and linear IgG and/or C3 deposit on direct immunofluorescence.
Cases with floor binding on direct immunofluorescence with presence of anti collagen 7 antibody is tested as a part of profile ELISA panel on profile ELISA were diagnosed as epidermolysis bullosa acquisita. The analysis excluded cases where anti-p200 pemphigoid was suspected, as immunoblots could not be done. The study was approved by the institute ethics committee (reference no IEC-INT/2022/Study-787, with ethics approval number: INT/IEC/2022/SPI-1866).
Results
A total of 92 cases of IgG-mediated sub-epidermal immunobullous diseases were diagnosed within this period, out of which nine were excluded (serology not performed or negative, or diagnosis not confirmed). The remaining cases were diagnosed as bullous pemphigoid (74, 89.2%), mucous membrane pemphigoid (8, 9.6%) and epidermolysis bullosa acquisita (1, 1.2%).
The mean age of patients was 60 (range 26–85) years with a male to female ratio of 1:1.4. Direct immunofluorescence revealed linear staining for IgG along basement membrane zone in all cases (100%). Staining for C3 was seen in 41 (49.4%) and IgA in 16 (19.3 %), all of which were studied for the presence of serrations. IgM antibody deposition was not detected in any case.
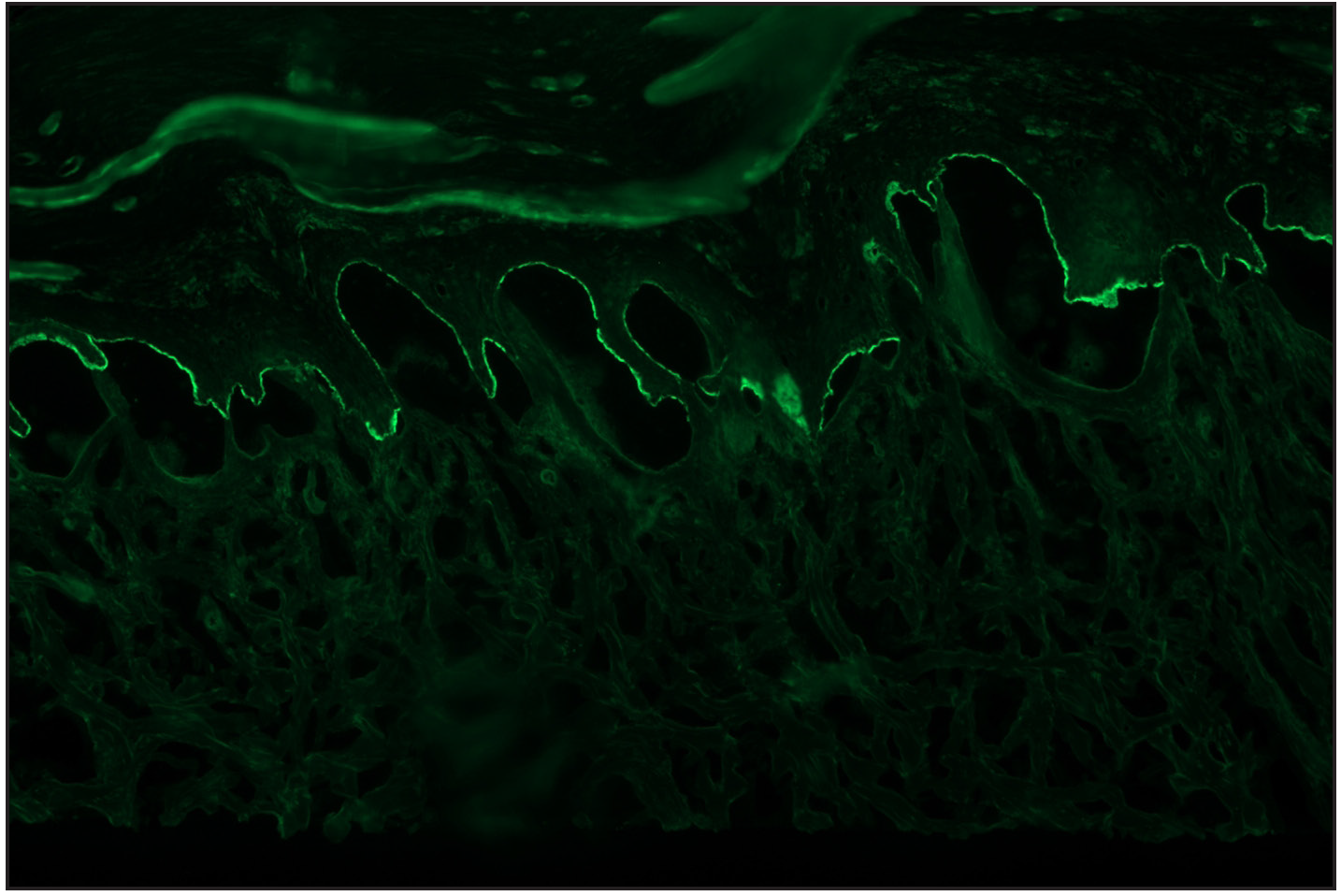
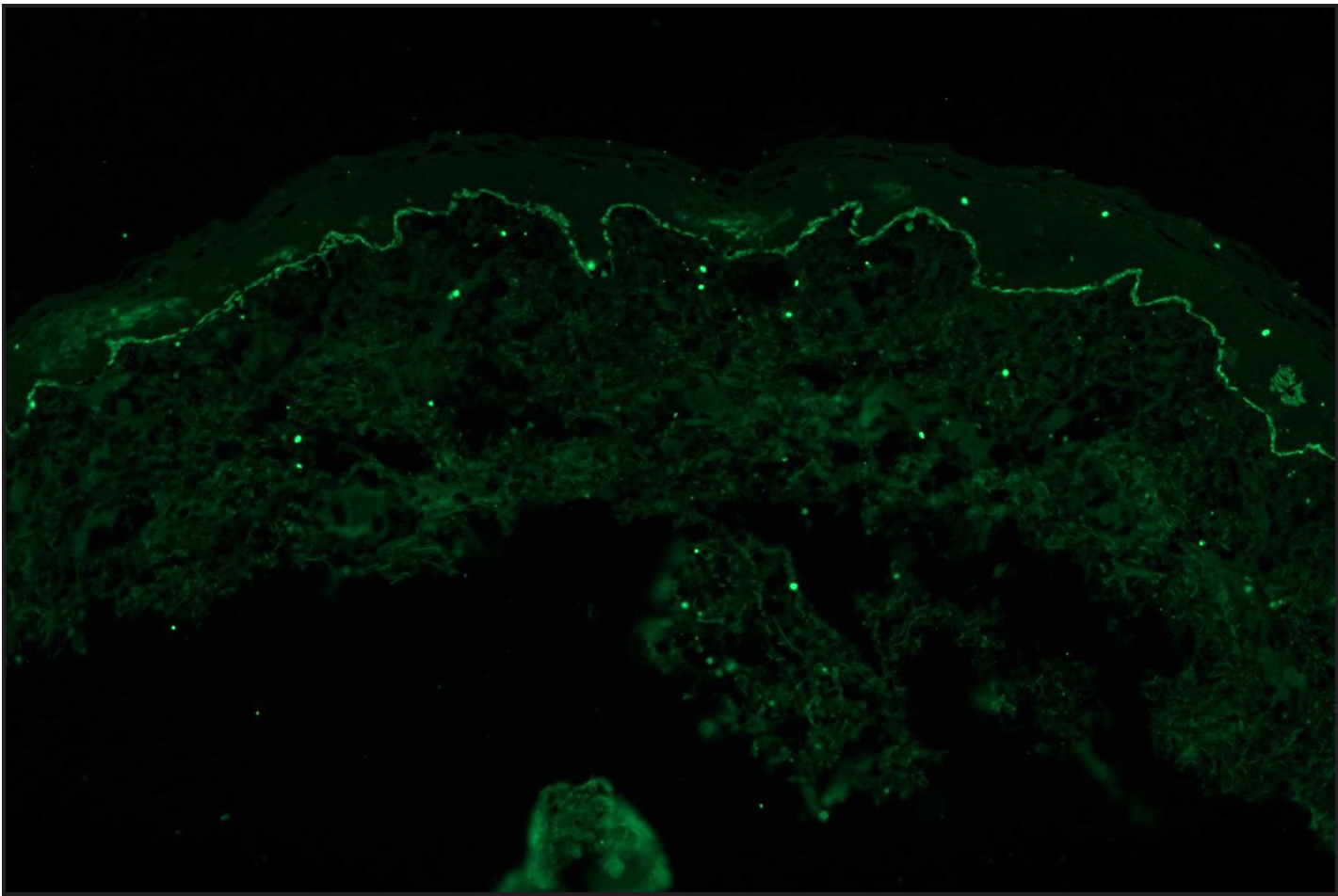
Among the pemphigoid group (BP and MMP) of patients, “n” serration pattern was observed in 66/82 (80.5%) cases. Out of the 74 bullous pemphigoid cases, 58 showed “n” serration pattern (78.4%). This pattern could also be interpreted on hair follicles [Figures 1a–f]. All eight cases of MMP displayed an “n” serration pattern (100%). The epidermolysis bullosa acquisita case showed “u” serration [Figures 2a–c]. In 16 (19.5%) cases, all bullous pemphigoid patients, serration pattern could not be ascertained due to faint intensity or complete sub-epidermal separation with limited intact direct immunofluorescence.
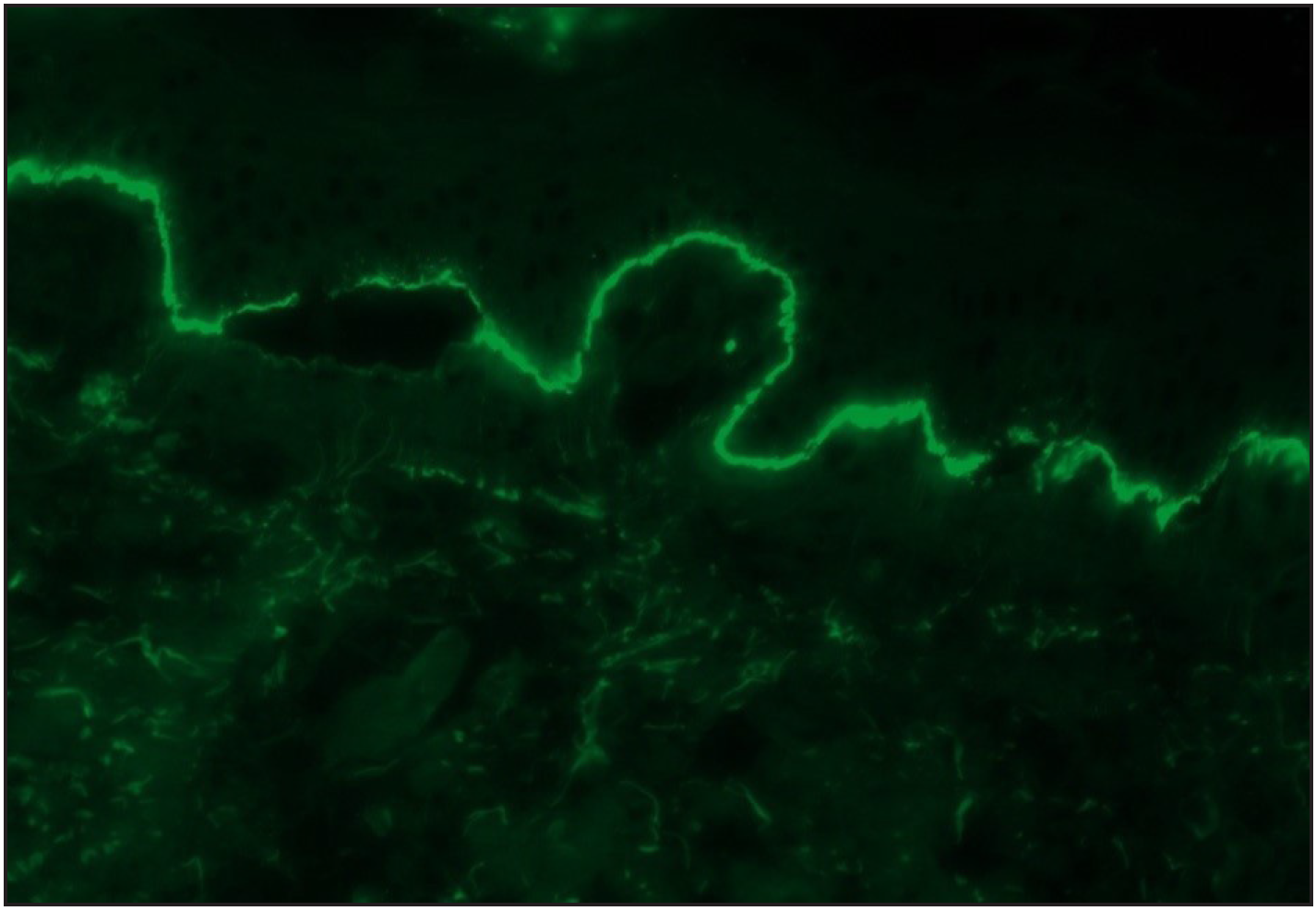
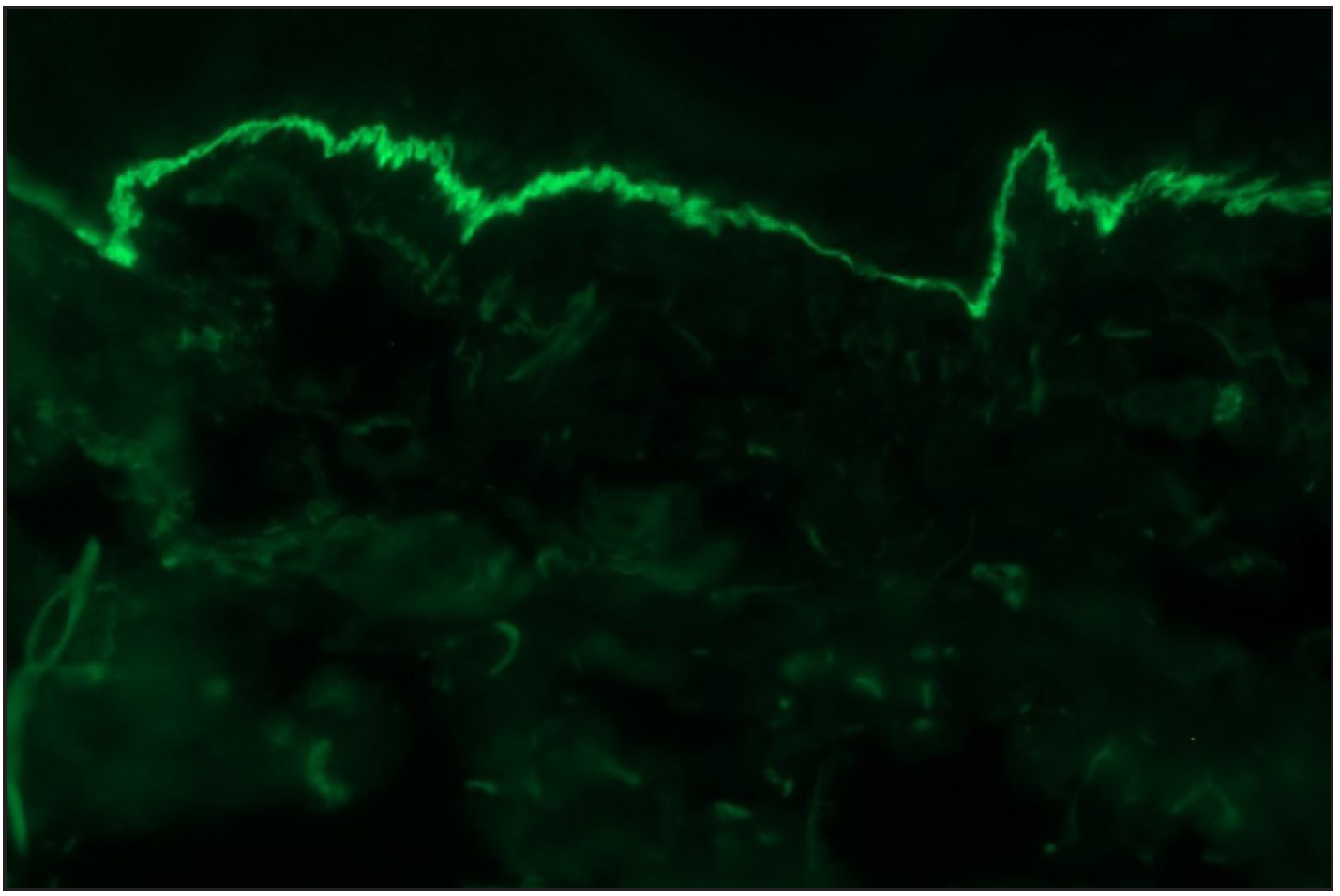
- A case of bullous pemphigoid, Direct immunofluorescence (DIF) showing linear IgG deposition along dermoepidermal junction (fluorescein isothiocyanate, ×200).
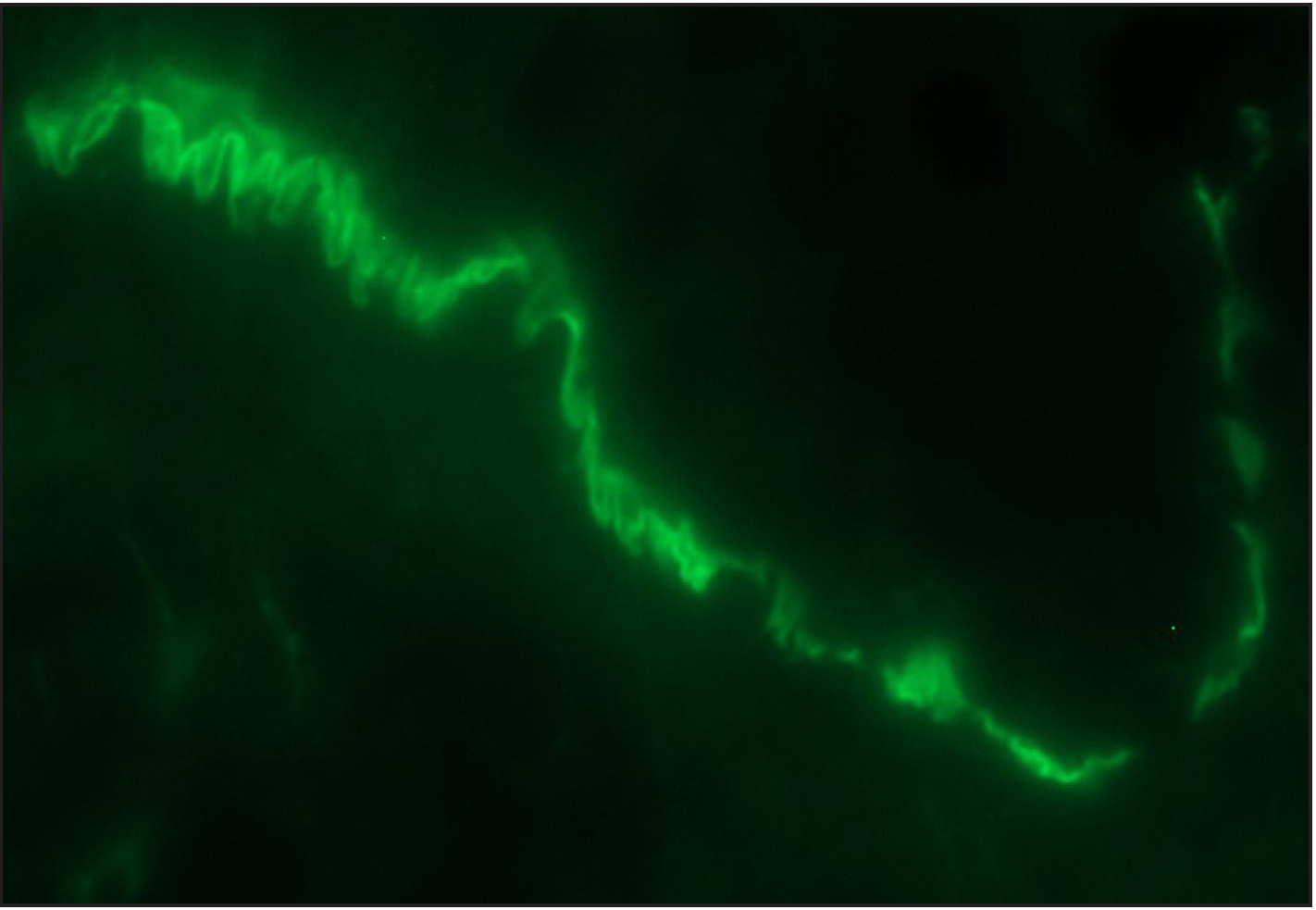
- Direct immunofluorescence (DIF) high power examination in a case of bullous pemphigoid showing “n” serrated pattern (fluorescein isothiocyanate, ×1000 under oil immersion)
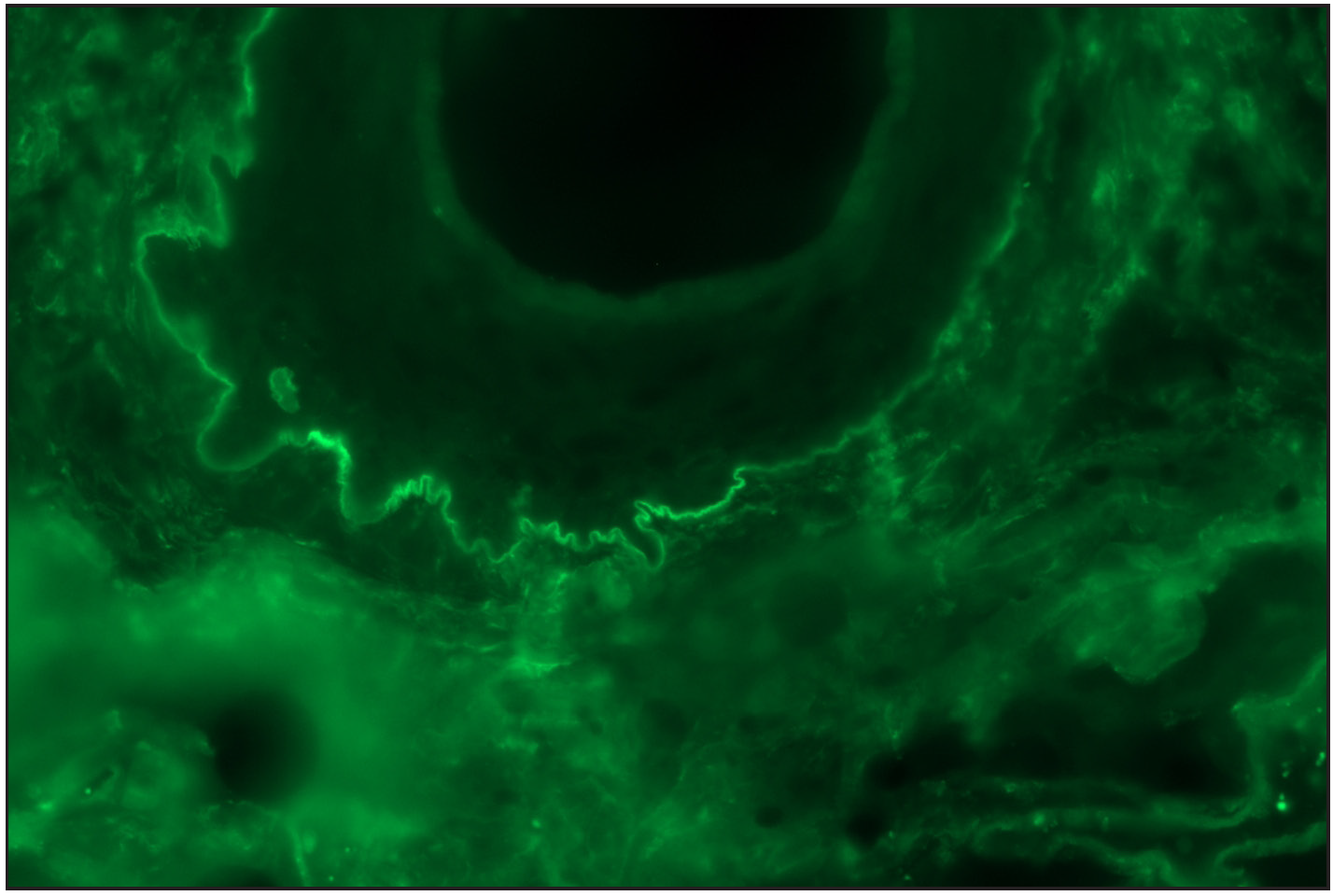
- ‘n’ serration pattern along hair follicle basement membrane on DIF in bullous pemphigoid (fluorescein isothiocyanate, ×400)
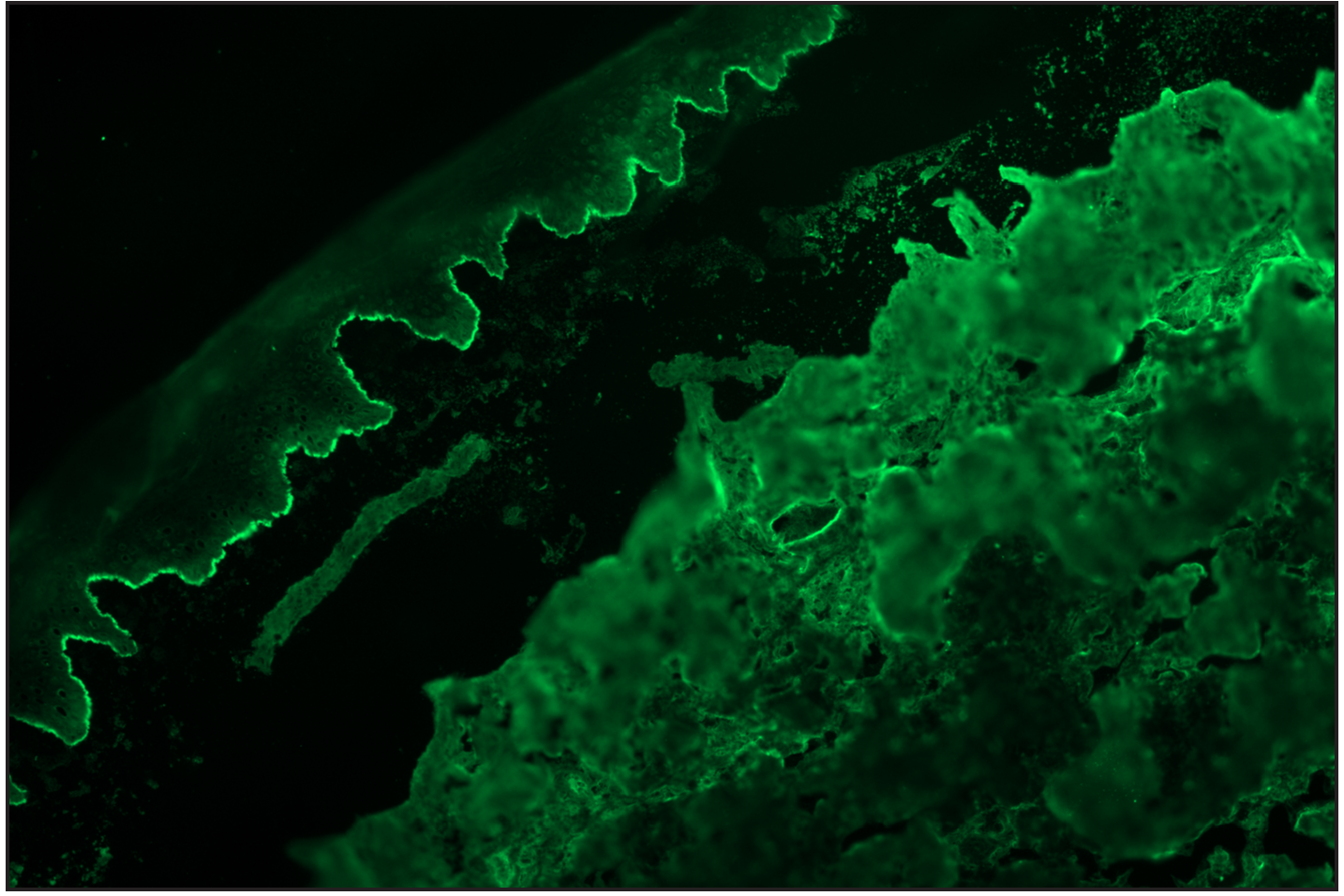
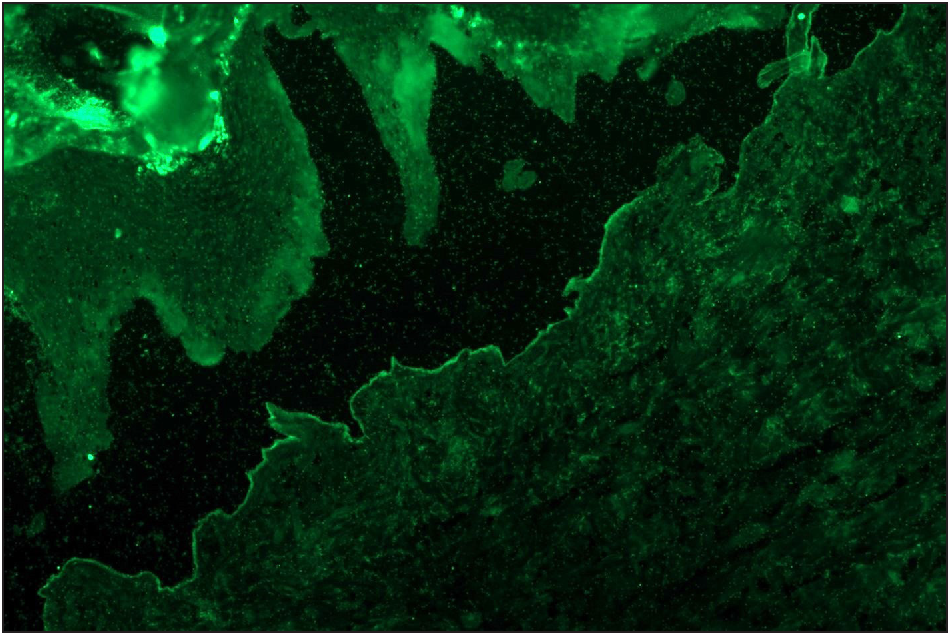
- Salt split skin (in house) indirect immunofluorescence of bullous pemphigoid shows roof binding of IgG (fluorescein isothiocyanate, ×200)
- BIOCHIP mosaic salt split (primate) shows roof binding of IgG in a case of bullous pemphigoid (fluorescein isothiocyanate, ×200)

- BP180 (Recombinant BP 180 NC16A) positivity on BIOCHIP mosaic in a case of bullous pemphigoid (fluorescein isothiocyanate, ×200).
- Direct immunofluorescence (DIF) in a case of epidermolysis bullosa acquisita (EBA) showing linear IgG deposition along dermoepidermal junction (fluorescein isothiocyanate, ×100)
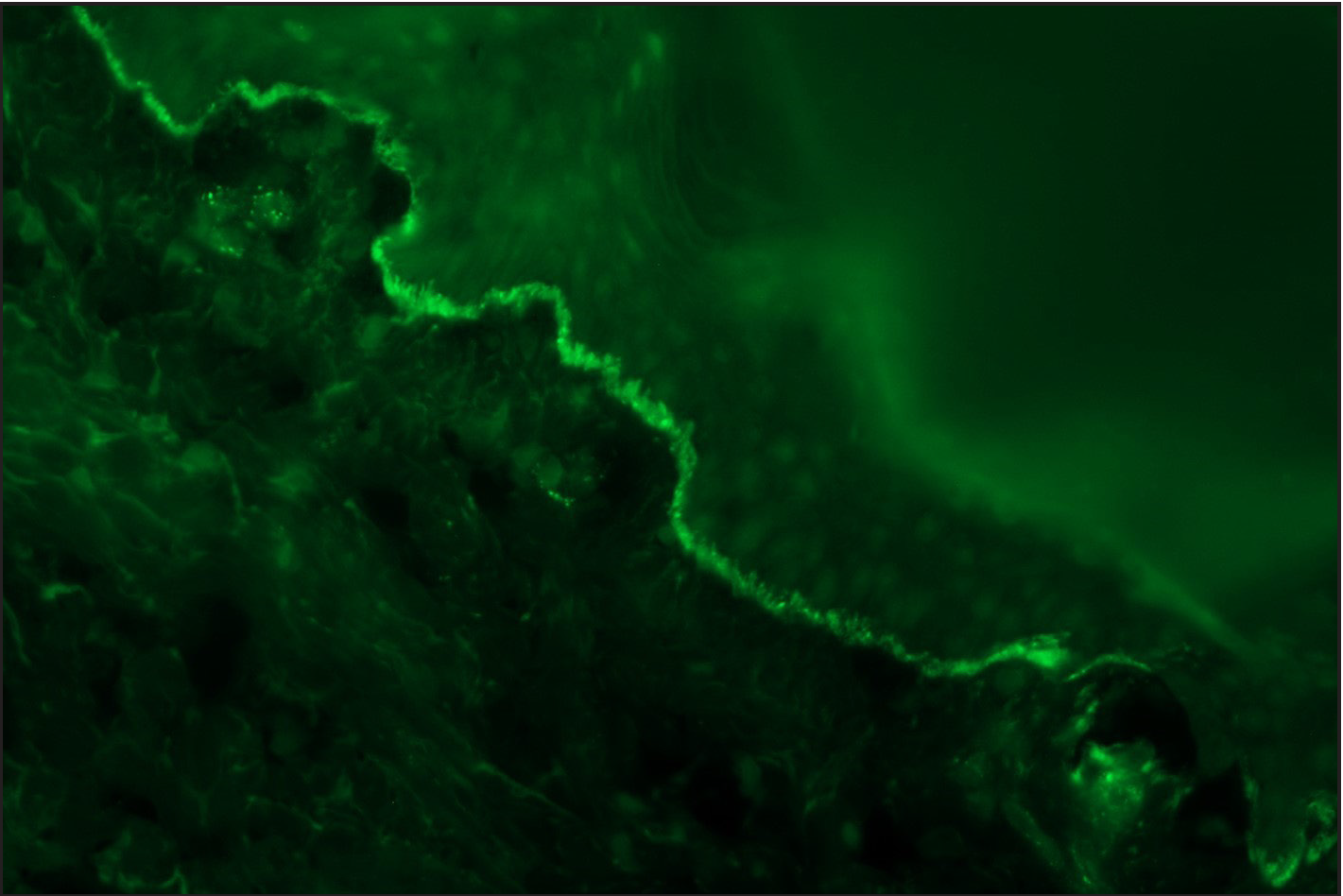
- DIF in a case of EBA showing ‘u’ serration pattern for IgG (fluorescein isothiocyanate, ×1000)
- Salt split skin (in house) indirect immunofluorescence of EBA case shows floor binding of IgG (fluorescein isothiocyanate, ×200)
Salt split skin indirect immunofluorescence was performed on 62 cases (61 cases of BP/MMP and one case of epidermolysis bullosa acquisita). In the bullous and membrane pemphigoid patients, roof binding was observed in 52 cases, and five cases showed both roof and floor binding. Four cases lacking any binding on salt-split skin indirect immunofluorescence showed positive for BP180 ELISA and displayed “n” serration on direct immunofluorescence. Salt-split skin indirect immunofluorescence was performed in six mucous membrane pemphigoid cases with roof binding in all. Other two cases of mucous membrane pemphigoid showed BP180 positivity. The case of epidermolysis bullosa acquisita showed only floor binding and was further confirmed on profile ELISA. Positive result for BP180 was seen in 73.8% (31/42), BP230 in 53.8% (7/13) while two cases (2/13, 15.4%) showed both BP180 and BP230 positivity using ELISA/BIOCHIP. There was no statistical association (using univariate analysis on two-by-two table) between the serration pattern and age, sex or BP180 positivity.
Discussion
Linear basement membrane zone IgG and C3 deposition are seen in bullous pemphigoid and other sub-epidermal immunobullous diseases necessitating serology for their distinction. However, factors such as sensitivity, technical expertise and higher cost of serological investigations such as ELISA and immunoblot impede their routine use. Approximately 50% of patients with epidermolysis bullosa acquisita and 20–50% with MMP lack circulating antibodies.9 Thus, epidermolysis bullosa acquisita can be misdiagnosed as bullous pemphigoid, especially when seronegative. Analysing direct immunofluorescence pattern on high power divides these sub-epidermal immunobullous diseases into two groups:
-
“n”-serrated pattern visualised in bullous pemphigoid, MMP, pemphigoid gestationis, linear IgA dermatosis and anti-p200 pemphigoid.
-
“u”-serrated pattern seen in epidermolysis bullosa acquisita, IgA epidermolysis bullosa acquisita and in some cases of bullous systemic lupus erythematosus.
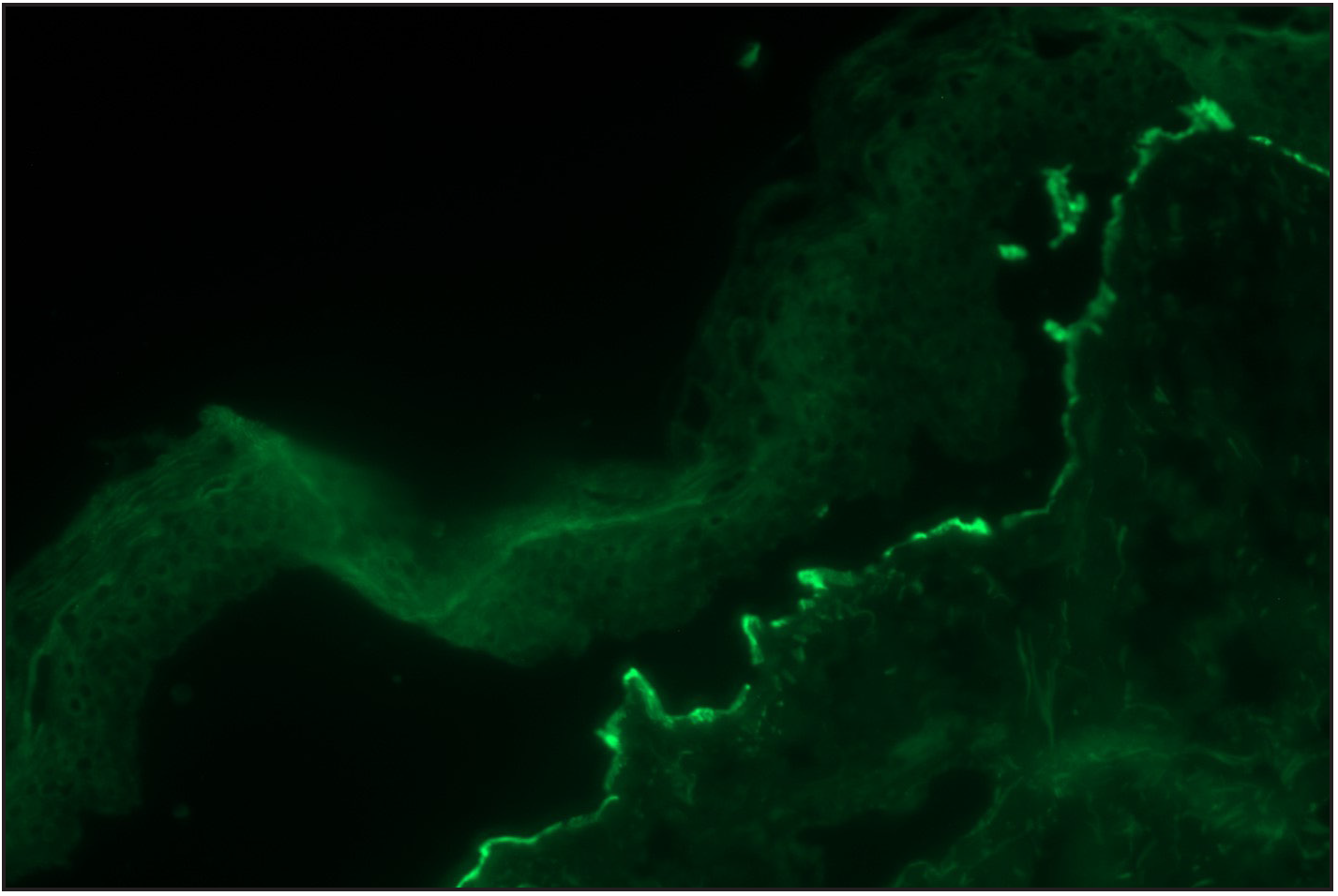
Arora et al. visualised “n” serrations in all nine cases of anti-p200 pemphigoid, which also exhibited dermal binding on salt split skin indirect immunofluorescence.12 Similarly, on analysing the mucous membrane pemphigoid group, Terra et al. reported “n” pattern in all 10 cases of antilaminin-332 membrane pemphigoid.13 Anti-laminin-332 MMP and anti-p200 pemphigoid are rare and show floor binding on salt split skin indirect immunofluorescence. Immunoblot is considered the gold standard for confirming anti-laminin 332 MMP or anti p-200 pemphigoid. Serration pattern analysis (n serration) with salt split skin indirect immunofluorescence (floor binding) is a good screening tool to suspect these entities. All the mucous membrane pemphigoid cases in our series tested for salt split skin indirect immunofluorescence showed roof binding or BP180 positivity, thus confirming to be classic mucous membrane pemphigoid. We could not carry out immunoblot to determine whether any of our eight cases of mucous membrane pemphigoid had anti laminin-332 MMP. Further, four cases of anti-p200 pemphigoid were suspected (“n” serration pattern on DIF and floor binding on SSS IIF), but were excluded due to lack of immunoblot [Figures 3a–c].
- Direct immunofluorescence (DIF) in a case of suspected anti p-200 pemphigoid showing linear IgG deposition along dermoepidermal junction (fluorescein isothiocyanate, ×200)
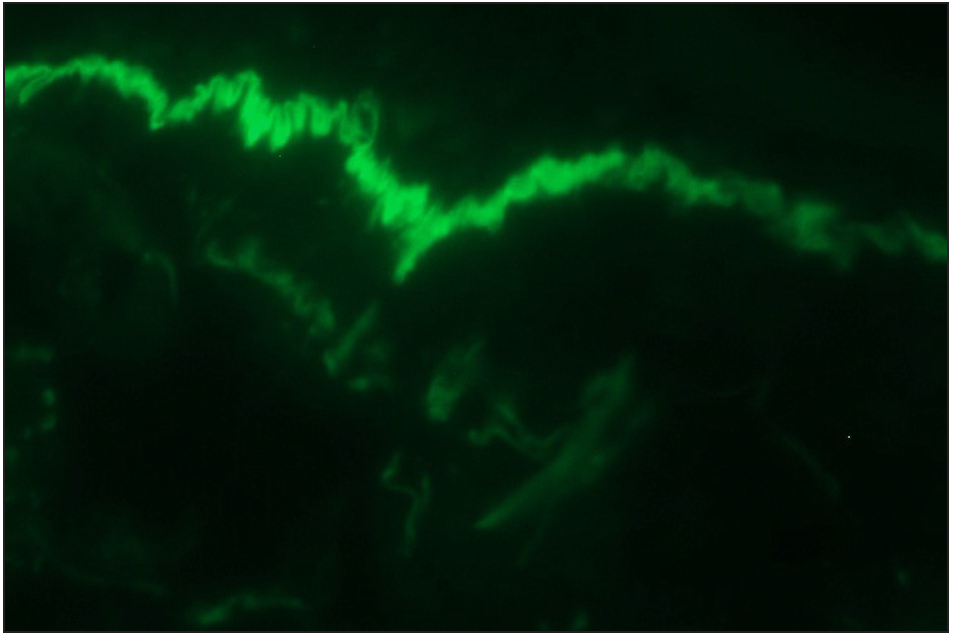
- Direct immunofluorescence (DIF) high power examination of the same case in Figure 3a showing “n” serrated pattern (fluorescein isothiocyanate, ×1000 under oil immersion)
- Salt split skin (in house) indirect immunofluorescence of the suspected anti-p200 pemphigoid shows floor binding of IgG (fluorescein isothiocyanate, ×200)
In our study, majority of the cases were of bullous and mucous membrane pemphigoids. We had only one proven case of epidermolysis bullosa acquisita (1 out of 83 IgG mediated sub-epidermal immunobullous disease, 1.2%). The extreme rarity of epidermolysis bullosa acquisita in our study population may be a reflection of low incidence of this disease in north India, compared to south Indian population, possibly due to genetic variation.12
Serration patterns of sub-epidermal immunobullous disease have been studied by various authors [Table 1], with detection rate ranging from 69.2–97.5%.3,4,12 A high detection rate suggests that a majority of bullous pemphigoid cases can be distinguished from other sub-epidermal immunobullous diseases successfully. It is a cost-effective tool that does not require extensive training or validation and can be practiced effectively in all laboratories where direct immunofluorescence facilities are available. Presence of roof (or roof plus floor) pattern of binding in salt split skin indirect immunofluorescence along with “n” serration pattern on direct immunofluorescence can be taken as diagnostic for bullous or mucous membrane pemphigoid, especially in resource-poor settings where ELISA, immunoblot or BIOCHIP may not be available. µm, microns; ELISA-BP180, enzyme-linked immunosorbent assay; SSS-IIF, salt split skin indirect immunofluorescence
Parameters
Serological diagnosis
Thickness of section
Rate of “n” serration detection in pemphigoid group
Present study (2023)
SSS-IIF, ELISA, BIOCHIP mosaic
5–6 µm
80.5%
Holtsche et al. (3)
SSS-IIF, ELISA, Immunoblot
6 µm; if not visualised then re-examined at 4-µm sections.
76%
Meijer et al. (4)
ELISA
6 µm
97.5%
Arora et al. (11)
SSS-IIF
6 µm
82.6%
Limitations
The study is limited by only single case of epidermolysis bullosa acquisita, lack of detailed serological investigations and immunoblot in all cases.
Conclusion
Serration pattern analysis is an easy-to-interpret and highly useful technique for the differential diagnosis of subepidermal immunobullous diseases.
Declaration of patient consent
Patient consent was not required as stored patient’s samples were used, and was exempted by the Institute Ethics Committee.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Serological diagnosis diagnosis of autoimmune bullous skin diseases. Front Immunol. 2019;10:1974-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comparison of three type VII collagen (C7) assays for serologic diagnosis of epidermolysis bullosa acquisita (EBA) J Am Acad Dermatol. 2016;74:1166-72.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value and practicability of serration pattern analysis by direct immunofluorescence microscopy in pemphigoid diseases. Acta Derm Venereol. 2021;101:1-5.
- [Google Scholar]
- Serration pattern analysis for differentiating epidermolysis bullosa acquisita from other pemphigoid diseases. J Am Acad Dermatol. 2018;78:754-59.
- [CrossRef] [PubMed] [Google Scholar]
- A new indirect immunofluorescence BIOCHIP method for the serological diagnosis of bullous pemphigoid: A review of literature. Australas J Dermatol. 2019;60:173-7.
- [Google Scholar]
- Serological diagnosis of autoimmune bullous skin diseases: Prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49-53.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Updated S2 K guidelines for the management of bullous pemphigoid initiated by the European Academy of Dermatology and Venereology (EADV) J Eur Acad Dermatol Venereol.. 2022;36:1689-1704.
- [CrossRef] [PubMed] [Google Scholar]
- Management of bullous pemphigoid: The European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015;172:867-7.
- [CrossRef] [PubMed] [Google Scholar]
- The diagnosis and blistering mechanisms of mucous membrane pemphigoid. Front Immunol. 2019;10:34-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. 2004;151:112-8.
- [CrossRef] [PubMed] [Google Scholar]
- The n- vs. u-serration is a learnable criterion to differentiate pemphigoid from epidermolysis bullosa acquisita in direct immunofluorescence serration pattern analysis. Br J Dermatol. 2013;169:100-5.
- [CrossRef] [PubMed] [Google Scholar]
- Serration pattern analysis as a practical adjunct tool for categorization of subepidermal autoimmune blistering diseases. Indian J Dermatol Venereol Leprol. 2021;87:778-86.
- [CrossRef] [PubMed] [Google Scholar]
- Immunofluorescence serration pattern analysis as a diagnostic criterion in antilaminin‐332 mucous membrane pemphigoid: Immunopathological findings and clinical experience in 10 Dutch patients. Br J Dermatol. 2011;165:815-22.
- [CrossRef] [PubMed] [Google Scholar]






