Translate this page into:
A low-grade well-differentiated neuroendocrine tumour originating from the skin
Corresponding author: Prof. Tao Qu, No. 1, Shuaifuyuan, Dongcheng District, Beijing, China. qutao2011@126.com
-
Received: ,
Accepted: ,
How to cite this article: Jia Q-N, Wang W-M, Zeng Y-P, Qu T. A low-grade well-differentiated neuroendocrine tumour originating from the skin. Indian J Dermatol Venereol Leprol. 2024;90:136. doi: 10.25259/IJDVL_1001_2022
Dear Editor,
Neuroendocrine neoplasms involve the gastrointestinal and bronchopulmonary systems most frequently.1 In 2019 the WHO categorized them as well-differentiated, poorly differentiated, and mixed neuroendocrine-non-neuroendocrine neoplasm. Well-differentiated neuroendocrine tumors are further classified into low, intermediate, and high-grade based on their mitotic and Ki-67 proliferation indexes. Low-grade, well-differentiated neuroendocrine tumors have a mitotic index <2/2 mm2 or Ki-67 index <3%. Here, we report a low-grade, well-differentiated neuroendocrine tumor originating from the skin with histological evidence of epidermotropism.
A 72-year-old man presented with an asymptomatic reddish tumor on his sternal region for two years. Physical examination revealed a 3 × 5 cm, poorly defined, crusted, reddish tumor with an infiltrative base [Figure 1a]. He opted for radiotherapy instead of surgical excision, and which minimally reduced his tumor size. Subsequently, he underwent Mohs micrographic surgery in our department. The patient reported a 10-year history of untreated meningioma. A PET-CT scan detected no other abnormality in his internal organs.
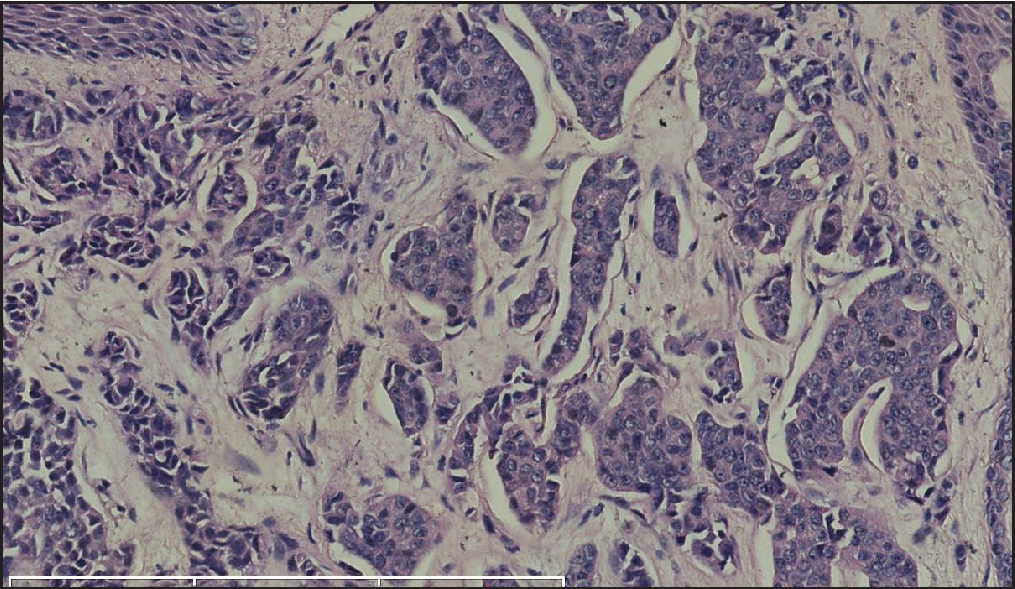
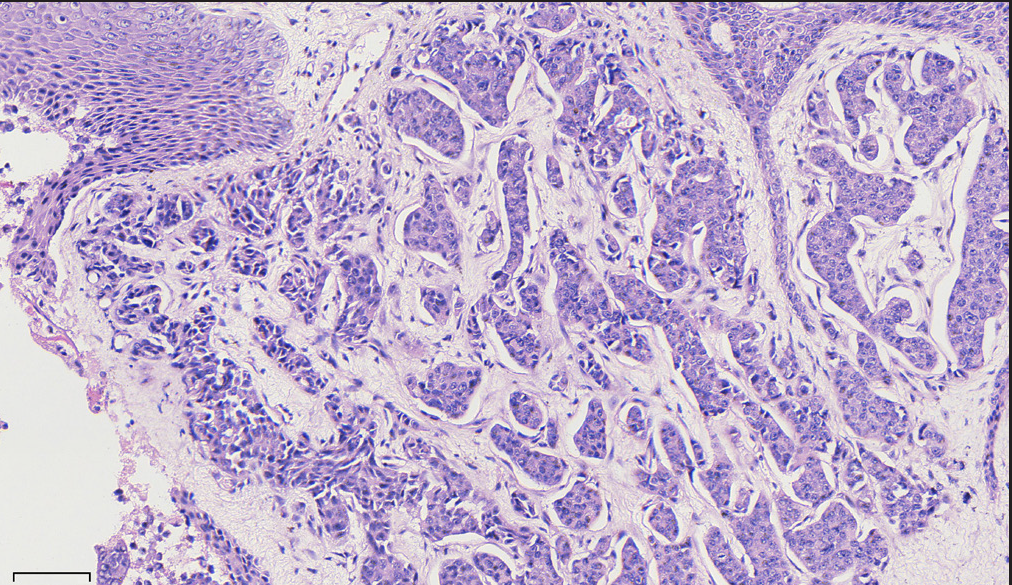
Histopathology revealed two types of tumor cells. One type possessed relatively abundant cytoplasm with uniform nuclei and was distributed in the superficial dermis. The other variant had small and hyperchromatic nuclei aggregated in a tight structure and occupied the mid and lower dermis [Figures 1b–1d]. Notably, tumor cells invaded the epidermis in a pagetoid growth pattern. Prominent retraction artifact was noted around tumor nests. No mucin deposition was detected. Immunohistochemical analysis of tumor cells was positive for NSE [Figure 2a], synaptophysin [Figure 2b], chromogranin A [Figure 2c], EMA, Ber-EP4, Bcl-2, and AE1/AE3, weakly positive for GATA3 and ER, and focally positive for CK7, but negative for S100 protein, CK20, p63, CK5/6, CD31, and TTF-1. Ki-67 proliferative index was less than 3% [Figure 2d]. Based on these findings, we diagnosed a low-grade well-differentiated cutaneous neuroendocrine tumor. No recurrence or metastasis was noted in the subsequent 9-month follow-up.
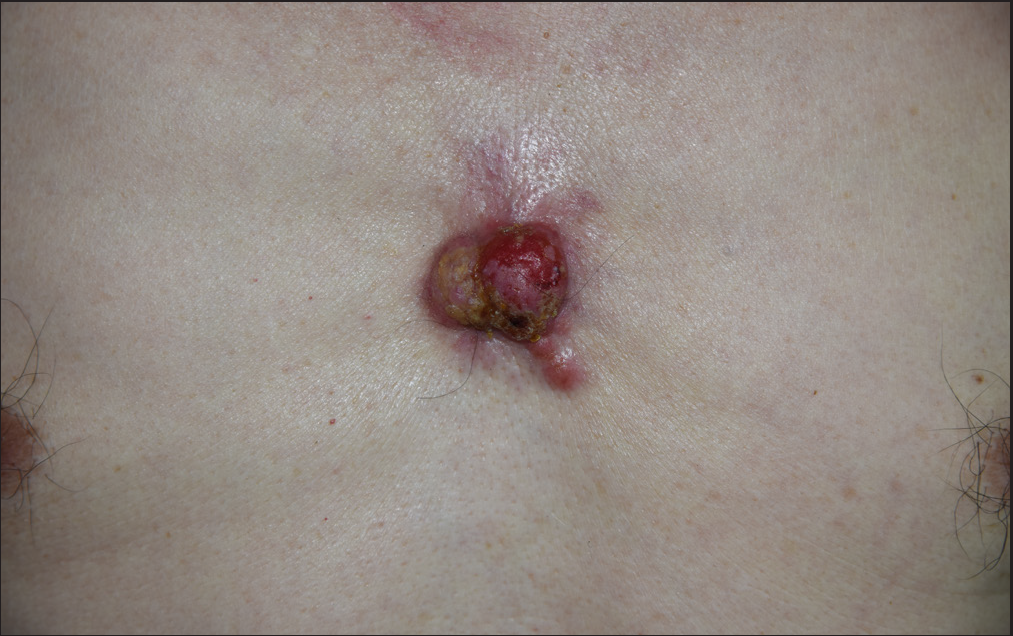
- A crusted, reddish tumor with an infiltrated base on the sternal region.
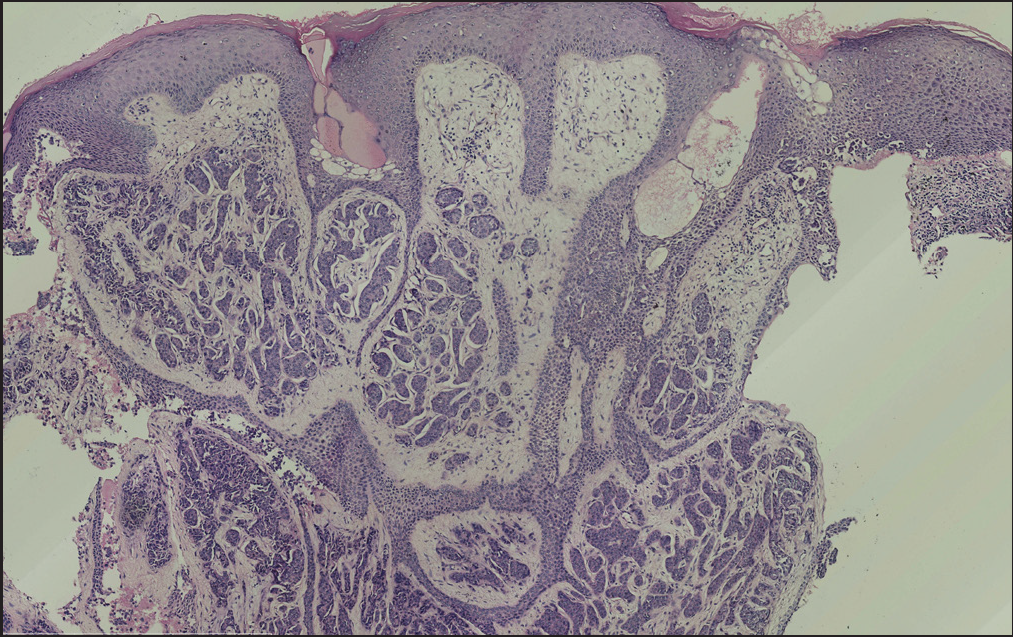
- An ill-defined neoplasm consisting of cell strands and cords (H&E; magnification).
- The trabecular structures were observed in cell nests (H&E; scale bar: 300 um).
- Polygonal or cuboidal tumor cells with round to oval nuclei and eosinophilic granular cytoplasm. (H&E; scale bar: 70 um).
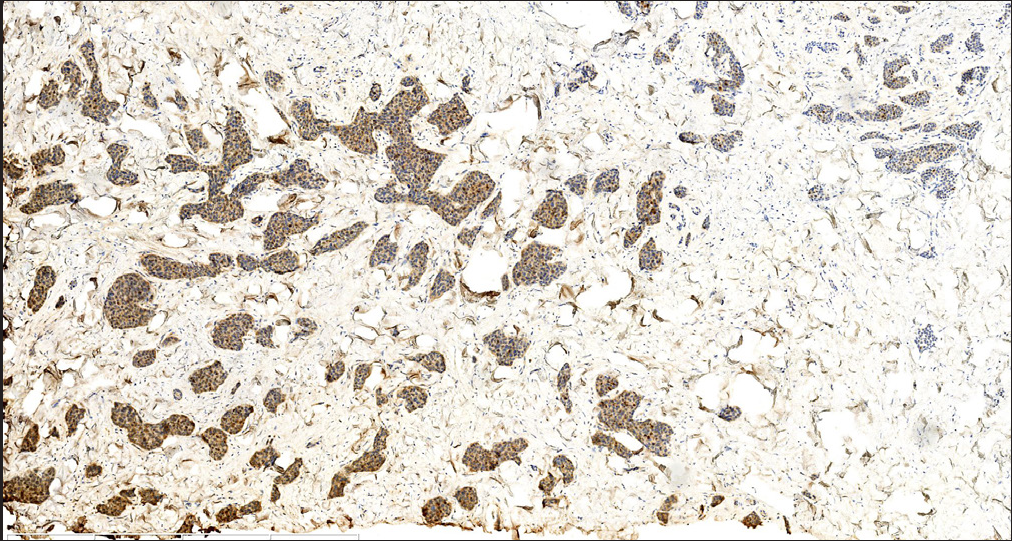
- Tumor cells were diffusely positive for neuron-specific enolase (NSE) (S-P; scale bar: 1 mm).

- Tumor cells were diffusely positive for chromogranin A (S-P; scale bar: 800 um).
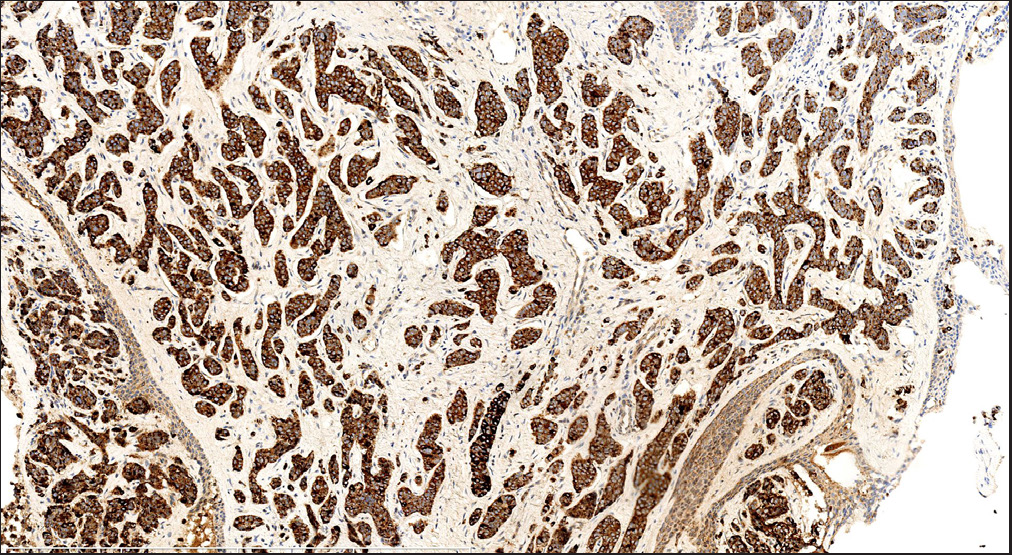
- Tumor cells were diffusely positive for chromogranin A (S-P; scale bar: 800 um).
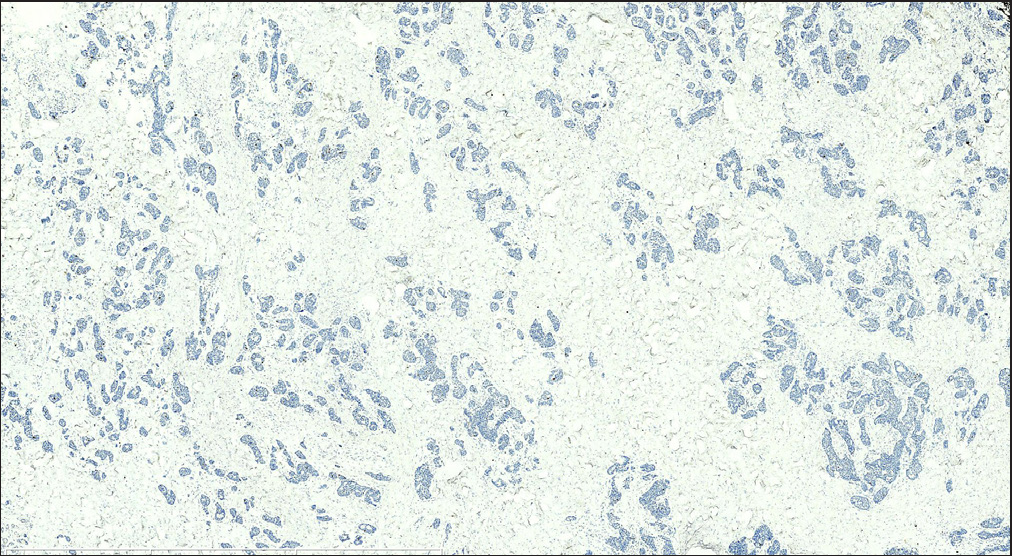
- Tumor cells had a Ki-67 proliferative index of less than 3% (S-P; scale bar: 3 mm).
Cutaneous neuroendocrine tumors often represent gastrointestinal and bronchopulmonary-system metastases and rarely originate from the skin. As metastatic and primary neuroendocrine tumors may have similar histopathological features, a comprehensive clinical work-up is necessary for the correct diagnosis. The present case, with no evidence of metastasis from other organs, suggested skin origin.
A clinicopathologic study has reported three cases of primary cutaneous carcinoid tumor and proposed “low-grade neuroendocrine carcinoma of the skin” as a distinct subtype of cutaneous neuroendocrine tumors. Its diagnostic criteria include a primary cutaneous tumor with neuroendocrine differentiation, positive immunohistochemistry for synaptophysin and chromogranin A, and low-grade cytology.1 Another study proposed “well-differentiated neuroendocrine tumors in skin” which included low and intermediate-grade carcinoid and neuroendocrine tumors arising from the skin, or as a part of metastasis.2 Recently, Goto et al3 performed a clinicopathologic study (n = 13) and hypothesized an apocrine/eccrine sweat gland origin and differentiation for this entity and thus renamed it “sweat-gland carcinoma with neuroendocrine differentiation.” So, the nomenclature of this tumor remains variable and confusing in the literature. More studies are needed to confirm its nature, origin, definition, and diagnostic criteria.
Well-differentiated and low-grade neuroendocrine tumors of the skin predominantly affect middle-aged to elderly males. The most common site is trunk, including the chest, abdomen, and inguinal regions. The most common clinical presentation is a solitary, firm, reddish to bluish-purple papule, nodule, or tumor. Histopathologically, it presents as cell islands, nests, and cords of variable size and shape and shows characteristic growth patterns, including trabecular and ribbon architectures. Cytologically, cells are usually polygonal, cuboidal, or columnar, with a round to oval nuclei and eosinophilic granular cytoplasm. Epidermotropism is rarely described in such cases, and we found only 11 cases in the literature displaying pagetoid growth pattern.1,3–5 On immunohistochemical analysis, tumor cells are usually positive for chromogranin A, synaptophysin, and NSE, consistent with our patient. Variable Immunoreactivity has been reported for CK7, CK19, EMA, AE1/AE3, CEA, Ber-EP4, GCDFP-15, GATA3, ER, PR, and Bcl-2. Although it is low-grade cytologically, it has an unsatisfactory prognosis and not considered an indolent tumor. Recurrence, regional, and non-regional lymph node metastases and distant organ metastasis to peritoneum, lung, and bone have been reported.
We considered various differentials such as Merkel cell carcinoma, basal cell carcinoma, and adnexal carcinomas, such as sebaceous carcinoma, primary cutaneous mucinous carcinoma, endocrine mucin-producing sweat gland carcinoma, microcystic adnexal carcinoma, syringocystadenoma. Merkel cell carcinoma is a classic cutaneous neuroendocrine malignancy. It exhibits more aggressive biological behavior and shows a predilection for sun-damaged skin. The characteristic histopathology demonstrates cells with large and pale nuclei containing tiny nucleoli. Numerous mitotic figures and necrotic areas are prominent, with high Ki-67 proliferative index. Moreover, it exhibits dot-like CK20 positivity in most cases. The present case’s clinical course, histopathological features, and immunohistological markers (CK20 negativity and low Ki-67 proliferative index) help in ruling out Merkel cell carcinoma. The retraction artifacts around cell nests may suggest basal cell carcinoma. However, it has an infiltrative growth pattern, and tumor nests consist basaloid cells in the dermis. It could be ruled out because tumor cells invaded the epidermis and did not express p63 or CK5/6 in the current case. Although sebaceous carcinoma may also display a carcinoid-like pattern, it usually occurs on the scalp and presents sebaceous differentiation and lack neuroendocrine markers. Primary cutaneous mucinous carcinoma is a rare, low-grade, invasive adnexal neoplasm with malignant biological behavior. It usually arises on the face, axilla, and trunk, especially the eyelids and scalp, and typically presents as an erythematous, asymptomatic nodule. The characteristic histopathological features are small tumor cell islands with abundant dermal mucin deposition, stained by alcian blue. Endocrine mucin-producing sweat gland carcinoma is a sweat gland carcinoma with neuroendocrine differentiation. It predominately affects the eyelid and periorbital skin of older women. Histopathologically, it has an expansive growth pattern with large solid or cystic nests and a distinctive feature of surrounding myoepithelial cells. Microcystic adnexal carcinoma exhibits a deeply infiltrative growth pattern: numerous keratinous cysts in the superficial dermis; islands or cords of basaloid and squamous cells in the mid dermis; cell nests and strands with perineural invasion in the deeper aspect. Syringocystadenoma papilliferum is a benign neoplasm of apocrine differentiation with tubular and papillary growth patterns. It usually presents on the scalp with concomitant nevus sebaceus. The lesions appear as grouped papules and nodules with scale-crust.
In conclusion, we describe a primary cutaneous low-grade well-differentiated neuroendocrine with relatively indolent biological behavior, low-grade cytology, and characteristic histopathologic features, and is a distinct entity from other cutaneous neuroendocrine tumors.
Glossary
WHO : World Health Organization
PET-CT : positron emission tomography-computed tomography
NSE : neuron-specific enolase
EMA : epithelial membrane antibody
Bcl-2 : B-cell lymphoma-2
GATA3 : GATA binding protein 3
ER : estrogen receptor
CK : cytokeratin
CD : cluster of differentiation
TTF-1 : thyriod transcription factor-1
References
- Low-grade neuroendocrine carcinoma of the skin (primary cutaneous carcinoid tumor) as a distinctive entity of cutaneous neuroendocrine tumors: a clinicopathologic study of 3 cases with literature review. Am J Dermatopathol. 2017;39:250-8.
- [CrossRef] [PubMed] [Google Scholar]
- Well-differentiated neuroendocrine tumors in skin: Terminology and diagnostic utility of cytokeratin 5/6 and p. 63. J Cutan Pathol. 2017;44:557-62.
- [CrossRef] [PubMed] [Google Scholar]
- Sweat-gland carcinoma with neuroendocrine differentiation (SCAND): a clinicopathologic study of 13 cases with geneuroendocrine tumor analysis. Mod Pathol. 2022;35:33-43.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidermotropic neuroendocrine carcinoma. Immunohistochemical differentiation from simulators, including malignant melanoma. J Cutan Pathol. 1991;18:120-27.
- [CrossRef] [PubMed] [Google Scholar]
- Case of low-grade neuroendocrine carcinoma of the skin presenting metastases to lymph nodes and peritoneum. J Dermatol. 2019;46:720-23.
- [CrossRef] [PubMed] [Google Scholar]





