Translate this page into:
Exacerbation of head and neck eczema with new-onset alopecia following dupilumab treatment in severe atopic dermatitis patients: A case series
Corresponding author: Dr. Young Bok Lee, Department of Dermatology, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Cheon Bo-ro, Uijeongbu, Korea. lyb80@catholic.ac.kr
-
Received: ,
Accepted: ,
How to cite this article: Koo HYR, Choi JY, Yu DS, Lee YB. Exacerbation of head and neck eczema with new-onset alopecia following dupilumab treatment in severe atopic dermatitis patients: A case series. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1018_2023
Dear Editor,
Atopic dermatitis (AD) is a chronic inflammatory skin disorder that may be comorbid with various autoimmune diseases, including alopecia areata (AA). Dupilumab, an interleukin (IL)-4 and IL-13 receptor antagonist, was approved by the FDA for the treatment of moderate-to-severe AD. Although some case reports have demonstrated improvement in AA with dupilumab, there are conflicting reports of patients developing new-onset hair loss during treatment.
Three of our severe AD patients, whose eczema area and severity index (EASI) scores were greater than 23, developed alopecic patches after treatment with dupilumab. None of the three patients had a history of hair loss, including AA. They presented with multiple, erythematous, scaly patches accompanied by conspicuous hair loss, which manifested between 8 and 12 weeks after treatment initiation [Figures 1a–1c]. Notably, the alopecic lesions primarily affected the parietal scalp regions in all patients; however, in the first patient, the hair loss rapidly progressed, encompassing the entire forehead. Interestingly, the patients manifested concurrent exacerbation of head and neck eczema, with head and neck EASI scores increasing from 2.5 to 4.5 in the first patient and from 1.0 to 1.8 in the other patients. The first two patients observed spontaneous hair regrowth in 2 to 3 months while continuing dupilumab. The third patient, nevertheless, discontinued dupilumab upon experiencing abrupt hair loss accompanied by erythema along the frontal hairline after 8 weeks of starting treatment. A punch biopsy from the scalp was subjected to histopathologic examination which revealed parakeratosis, irregular acanthosis and marked spongiosis with perivascular lymphocytic infiltration predominantly in the papillary dermis, resembling subacute eczematous dermatitis [Figures 2a–2c]. After discontinuation of dupilumab, the patient received systemic steroids and cyclosporine, resulting in the gradual emergence of vellus hairs.
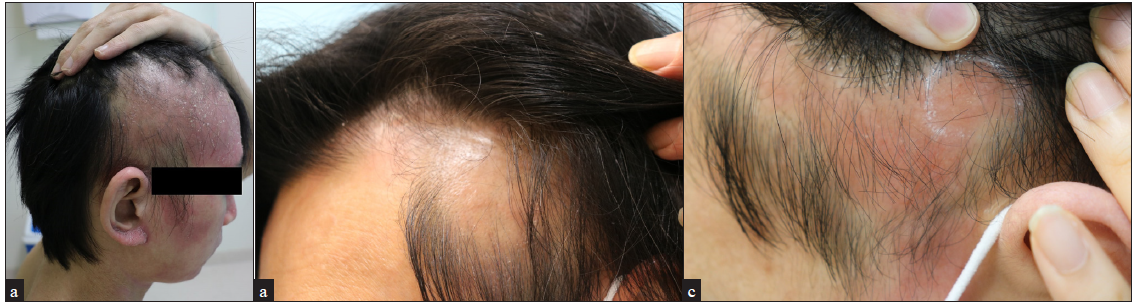
- Three men with severe atopic dermatitis (a) 28-year old, (b) 47-year old and (c) 25-year old, presented with erythematous, diffuse, non-scarring alopecic patches on the frontal and parietal scalp following the use of dupilumab.

- Haematoxylin and eosin stained tissue sectioned showing parakeratosis, irregular acanthosis, and noticeable spongiosis with perivascular, lymphocytic infiltratio mainly in the papillary dermis, resembling subacute eczematous dermatitis. Note there is no terminal hair or increased catagen/telogen hairs. (a) 40x, (b) 100x and (c) 400x magnification.
Multiple case reports and clinical studies suggest that dupilumab may act as a novel remedy for AA. It has been proposed that dupilumab could treat Th2-associated inflammation in the hair follicles and promote hair growth.1 Nonetheless, there are multiple reports of patients developing alopecic patches while on dupilumab.2–7 These differences in clinical outcomes emphasise the importance of understanding the pathogenic mechanisms of hair loss related to Th2-associated pathways. Despite several studies reporting de novo AA arising after dupilumab treatment, only a few studies have performed histopathology examination, making a definitive diagnosis is uncertain. As summarised in Table 1, histopathologic findings of previous reports have revealed various findings of hair loss that are consistent with various disease entities, including AA, eczema, or drug-induced hair loss.2–7 Given that the hair loss observed in our cases appeared 2 to 3 months after the initiation of dupilumab, there appears to be a temporal relationship between the two events. Clinically, our patients exhibited hair loss with aggravation of eczema in the head and neck regions. The histopathological examination of one case showed features resembling subacute eczematous dermatitis, rather than the classic AA findings of dense peribulbar lymphocytic infiltrates.
| Sources | Age/Sex | Site and morphology | Latency period (Weeks) | Histopathological findings |
|---|---|---|---|---|
| Flanagan, 20192 | 27/M | Diffuse pink background erythema with ill-defined areas of non-scarring alopecia on the crown and temporal scalp | 18 |
|
| Barroso-García, 20184 | 31/M | Patches of hair loss on the anterior scalp | 6 |
|
| Salgüero-Fernández, 20195 | 33/M | Diffuse alopecia of the scalp, predominantly on the frontal and occipital areas, associated with erythema and scaling and areas of alopecia on the beard area | 7 |
|
| Zhu, 20206 | 31/M | Scalp, frontotemporal, localised nonscarring alopecic patch with perifollicular scale | 40 |
|
| Maiolini, 20217 | 22/M | Scaling, erythematous alopecia plaque, with pruritus, on the vertex region, 5 cm in diameter, with erythema-eczema pattern on dermoscopy | 20 |
|
| Our case, 2023 | 25/M | Hair loss with erythema along the frontal hairline | 8 |
|
We present three cases of patients with new-onset hair loss while on dupilumab for AD. This suggests that hair loss following dupilumab treatment may be a manifestation of exacerbated head and neck eczema with acute hair loss. Since only a limited number of histopathological examination of biopsy specimens have been performed, it is imperative for dermatologists to be aware of these phenomena and conduct scalp biopsies to guide treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that they have used artificial intelligence (AI)-assisted technology to assist in the writing or editing of the manuscript or image creations.
References
- Phase 2a randomized clinical trial of dupilumab (anti‐IL‐4Ra) for alopecia areata patients. Allergy. 2022;77:897-906.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Drug-induced alopecia after dupilumab therapy. JAAD Case Rep. 2019;5:54-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dupilumab and alopecia: Causative or therapeutic? Dermatol. 2019;235:306-7.
- [CrossRef] [PubMed] [Google Scholar]
- Alopecia areata in severe atopic dermatitis treated with dupilumab. J Investig Allergol Clin Immunol. 2018;28:420-1.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatitis and alopecia in a patient treated with dupilumab: A new adverse effect? Clin Exp Dermatol. 2019;44:e41-e43.
- [CrossRef] [PubMed] [Google Scholar]
- Inflammatory alopecia in patients on dupilumab: A retrospective cohort study at an academic institution. J Eur Acad Dermatol Venereol. 2020;34:e159-e161.
- [CrossRef] [PubMed] [Google Scholar]
- Alopecia areata-like and psoriasis after dupilumab use for atopic dermatitis. An Bras Dermatol. 2021;96:634-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






