Translate this page into:
Macrolide and fluoroquinolone resistance associated mutations in Mycoplasma genitalium in men who have sex with men attending STI clinic: A pilot study from India
Corresponding author: Dr. Benu Dhawan, Department of Microbiology, All India Institute of Medical Sciences, India. dhawanb@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Biswal D, Gupta S, Sethi S, Singh S, Khanna N, Dhawan B. Macrolide and fluoroquinolone resistance associated mutations in Mycoplasma genitalium in men who have sex with men attending STI clinic: A pilot study from India. Indian J Dermatol Venereol Leprol. 2024;90:632-5. doi: 10.25259/IJDVL_933_2023
Abstract
Background
Increasing rates of macrolide and fluroquinolone resistance in Mycoplasma genitalium (MG) are being reported worldwide with resultant treatment failure.
Aim
We aimed to determine the level of antibiotic resistance of MG in men who have sex with men (MSM) attending a sexually transmitted infections (STIs) clinic in New Delhi, India.
Methods
Real-time polymerase chain reaction (PCR) assays targeting MgPa and pdhD genes were performed to detect MG rectal, urogenital or oropharyngeal infections in 180 MSM between January 2022 and June 2023. Macrolide resistance–associated mutations (MRM) and quinolone resistance–associated mutations (QRM) were detected by specific amplification of domain V of 23SrRNA gene and appropriate regions of parC and gyrA genes respectively followed by sequencing. PCR-based screening for Chlamydia trachomatis (CT) infection was also performed.
Results
A total of 13 (7.2%) MSM were positive for MG infection. The most common site of infection was anorectum (8/13; 61.5%) followed by the urethra (5/13; 38.5%). None of the patients had infection at both the sites, and no oropharyngeal MG infection was detected. CT infection was detected in 37 (20.6%) MSM. Of the 13 MG-infected MSM, 6 (46.2%) were co-infected with CT. MRM and QRM were found in five (46.2%) and two (15.4%) strains, respectively. Both Quinolone resistance mutation (QRM)-harbouring strains also harboured MRM. All the five MG isolates carried the MRM A2071G. Both the QRM isolates co-harboured the parC and gyrA single-nucleotide polymorphisms. There was no correlation between the presence of antibiotic resistance and co-infection with CT (P = 0.52).
Limitation
Because all patients in the study were MSM, the high rate of resistance to macrolides and fluoroquinolones could not be extrapolated for non-MSM patients.
Conclusion
This is a report of an initial survey of antibiotic resistance to MG in a country where its diagnosis and treatment are not routinely available. We found a high prevalence of MG-carrying MRM, QRM and dual-class resistance in MSM in the absence of antibiotic exposure. This study mandates the need for both screening and detection of antimicrobial resistance against MG.
Keywords
Mycoplasma genitalium
Men who have sex with men
Macrolide resistance
Fluroquinolone resistance
Introduction
Mycoplasma genitalium (MG), an emerging pathogen of sexually transmitted infections (STIs), is an important cause of non-chlamydial, non-gonococcal urethritis and is associated with other urogenital conditions in both men and women. MG is underdiagnosed because its detection relies necessarily on molecular methods. Due to it being intrinsically resistant to antibiotics active at the cell wall, limited treatment options are available for MG infection. Recommended treatment is azithromycin and second-line agent is moxifloxacin. Unfortunately, there has been a recent increase in resistance to both antibiotics, resulting in treatment failure.
MG has an extraordinary capacity to develop antimicrobial resistance due to DNA mutations, which can be detected by molecular methods. Macrolide resistance is caused by mutations in the domain V of 23SrRNA gene. Resistance to fluoroquinolones are mainly associated with mutations in gyrA and parC genes.
According to a recent meta-analysis,1 the rate of macrolide resistance–associated mutations (MRM) in MG was 35.5%, with rates increasing from 10.0% in 2010 to 51.4% in 2017. Resistance to macrolides was found to be higher in the Western Pacific region and America as compared to Europe. Quinolone resistance–associated mutations (QRM) increased from 4.8% before 2010 to 9.3% in 2016–17. Overall, the prevalence of dual-class mutations was 2.8%, which did not change significantly over time and with regards to the geographical region.
A previous study from All India Institute of Medical Sciences, New Delhi reported a high prevalence of MG in symptomatic men who have sex with men (MSM).2 However, antibiotic resistance testing of MG was not performed.
With this in mind, we aimed to determine the level of antibiotic resistance of MG in MSM attending the STI clinic of Department of Dermatology and Venereology of the All India Institute of Medical Sciences, New Delhi, India.
Methods
All consecutive symptomatic MSM, who attended the STIs clinic, AIIMS, New Delhi, between January 2022 and June 2023, were included in the study. This study was approved by the institute’s research ethics committee (IEC-207/11.04.2020, RP-47/2021). First-void urine, rectal and oropharyngeal swabs were collected. All MSM were routinely screened for Chlamydia trachomatis (CT). To detect CT infection, a conventional PCR was performed targeting the ompA gene.3 In addition to CT screening, MSM were also tested for MG by two different real-time PCR assays targeting MgPa and pdhD genes, respectively, as described previously.4,5 MG infection was diagnosed in a sample only when both PCR results were positive.
MRM and QRM in MG were detected by specific amplification of domain V of 23SrRNA gene [Figure 1a] and relevant regions of parC [Figure 1b] and gyrA [Figure 1c] genes, respectively,6,7 followed by sequencing, which was carried out on an ABI PRISM 310 Genetic Analyser (PE Biosystems, Foster City, California) using a BigDye DNA sequencing kit (PE Biosystems, Foster City, California) according to the manufacturer’s instructions.
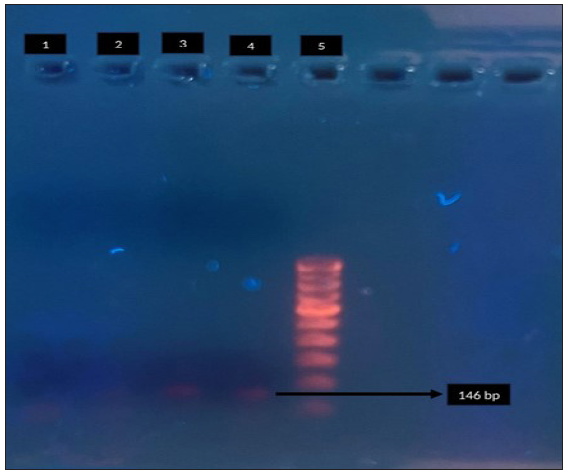
- Agarose gel electrophoresis for detection of macrolide resistance (23S r RNA) in Mycoplasma genitalium by polymerase chain reaction. Lane 1: negative control, Lane 2: clinical sample - negative, Lane 3: clinical sample - positive, Lane 4 - positive control, Lane 5: 100-bp DNA ladder. Black arrow indicates positive control for 23 S r RNA (146 bp).
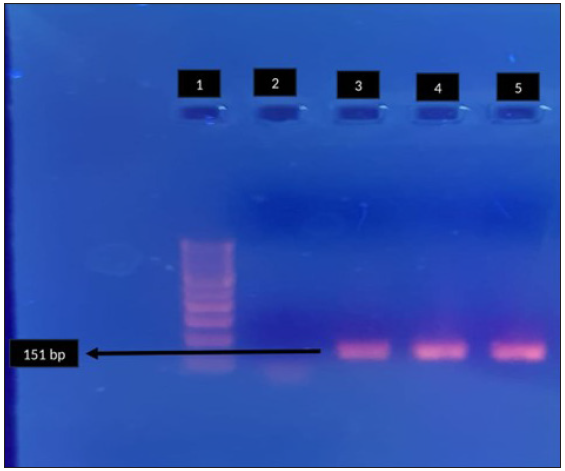
- Agarose gel electrophoresis for detection of fluroquinolone resistance (gyrA) in Mycoplasma genitalium by polymerase chain reaction. Lane 1: 100-bp DNA ladder, Lane 2: negative control, Lane 3, 4: clinical sample - positive, Lane 5: positive control. Black arrow indicates positive clinical sample for gyrA (151 bp).
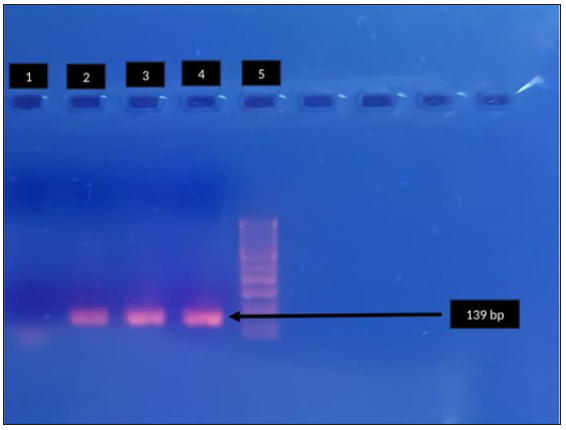
- Agarose gel electrophoresis for detection of fluroquinolone resistance (parC) in Mycoplasma genitalium by polymerase chain reaction. Lane 1: negative control, Lane 2, 3: clinical sample - positive, Lane 4: positive control, Lane 5: 100-bp DNA ladder. Black arrow indicates positive control parc (139bp).
Results
A total of 180 MSM were enrolled during the study period. MRM and QRM in MG were detected by nested PCR of domain V of 23 S r RNA [Figure 1a] and relevant regions of par C [Figure 1b] and gyr A [Figure 1c] respectively. The amplified genes were further sequenced on an ABI PRISM 310 Genetic Analyser (PE Biosystems, Foster City, California) using a Big Dye DNA sequencing kit (PE Biosystems, Foster City, California) according to the manufacturer’s instructions. The mean age of the study population was 30.6 years, and 71 (39.4%) were HIV-1 seropositive. Of the 180 patients enrolled, 106 (58.9%) were symptomatic and 74 (41.1%) were asymptomatic.
A total of 13 (7.2%) MSM were positive for MG infection. The most common site of infection was anorectum (8/13; 61.5%) followed by the urethra (5/13; 38.5%). None of the patients had infection at both the sites, and no oropharyngeal MG infection was detected. A total of 37 (20.6%) out of 180 MSM were positive for CT infection. Of the 13 MG-infected MSM, 6 (46.2%) were co-infected with CT.
Of the 13 MG strains, macrolide-resistant point mutation (A2071G) was detected in 46.2% (5/13) of the samples. Macrolide-resistant mutation was detected in 60% (3/5) rectal and 40% (2/5) of urogenital samples. None of the patients had taken prior azithromycin in the past 1 month. All 13 isolates were also analysed for QRM. Two of the 13 isolates (15.4%) harboured both parC and gyrA mutations, one each in rectal and urogenital sample, respectively. Single nucleotide polymorphisms (SNPs) were detected in both these isolates. None of the patients had taken prior fluoroquinolone treatment. Of note, both the QRM-harbouring strains also harboured MRM. There was no correlation between the presence of antibiotic resistance and co-infection with CT (P = 0.52).
Of the 13 MG infected patients who were treated with azithromycin, 5 of them did not respond. These were confirmed as macrolide resistant on sequencing. The macrolide resistant cases were then treated with moxifoxacin.
Three out of these five patients did not respond to moxifloxacin and then further confirmed as fluroquinolone resistant on sequencing. These patients were then treated with oral doxycycline for 7 days after which the symptoms resolved.
Discussion
Antimicrobial resistance in MG has become a global threat. This study, we believe, is the first to document the extent of macrolide and quinolone resistance in an Indian MSM population. We found a high prevalence of MG carrying MRM (46.2%), QRM (15.4%) and dual-class resistance-associated mutation (15.4%) in MSM. Macrolides resistance is usually much higher than that of fluroquinolones as reported in previous studies.1 Emergence of macrolide and quinolone resistance is associated with past exposure to these antibiotics as these are frequently used in CT, urinary tract and respiratory tract infections. However, none of the patients in the study had a history of macrolide or fluoroquinolone therapy in their recent past. Also, there was no correlation between the presence of antibiotic resistance and co-infection with CT. As reported by de Salazar et al.,8 there was a significant association between macrolide-resistant MG infection and being MSM with another STI in the last year, while no such associations was associated with fluoroquinolones resistance. The relatively high rate of macrolide- and fluroquinolone-resistant MG in the patients of the present study in the absence of their usage is disturbing and indicates other, yet to be established, factors contributing to their emergence.
MRM including A2071G, A2071T, A2072G are the most frequently detected mutations.9 In our study, all the five MG isolates carried the MRM A2071G. Both the quinolone-resistant MG strains co-harboured the parC and gyrA mutations. This not only limits the use of moxifloxacin-based therapy but also sitafloxacin, an alternate antibiotic, approved in some countries such as Japan for treating patients infected with MG strains harbouring only parC mutation. Moreover, sitafloxacin is not effective against MG isolates carrying both parC and gyrA mutations. Thus, this mandates monitoring of both parC and gyrA mutations as markers of quinolone treatment failure.
Studies have reported prevalence rate ranging from 6.1 to 68.3%,10 in the context of dual-class resistance. In our study, the prevalence of MG strains with dual-class resistance was 15%, which was in concordance with the previous study.10 This is concerning, considering that there is no established effective treatment for dual-class resistant MG. The combination therapy of doxycycline with sitafloxacin or pristinamycin has shown efficacy for treatment of dual-class resistant infections. However, both sitafloxacin and pristinamycin are not licensed for usage in India. Hence, both our patients with dual-class resistant infections were treated with doxycycline monotherapy for 1-week duration, after which their symptoms resolved.
Further research is required on regimens involving doxycycline combination therapy, a cheaper antibiotic with better potential or other antibiotics as an alternative for treatment of this infection. Also, pristinamycin, a novel antibiotic which is considered a third-line option in other countries, should be made available in India.
This is a report of an initial survey of antibiotic resistance in MG in a country where its diagnosis and treatment are not routinely available. Our data reveal a relatively high prevalence of MG in MSM, associated with high levels of antimicrobial resistance, furthermore implying that dual class–resistant strains are already in circulation in India. The high prevalence of dual class–resistant strains of MG in our study necessitates the need for the availability of third-line antibiotic options in India. Similar to our findings, there was no correlation between the presence of antibiotic resistance for either macrolides or fluroquinolones and co-infection with CT.10
Limitations
Our study had some limitations. First, the test of cure for MG was not performed. Second, because the study was conducted on MSM population, the high rate of resistance to macrolides and fluroquinolones could not be extrapolated for patients other than MSM. Thirdly, amino acid changes in gyrA and parC genes were not determined, which nonetheless, would have a minor impact on fluoroquinolone resistance. Lastly, small sample size is another limitation.
Conclusion
To conclude, this study has highlighted the importance of screening of MSM for MG and testing for antimicrobial resistance. Furthermore, treatment should be undertaken keeping in mind its high resistance rates. Moxifloxacin-based therapy may not be sufficient, which reiterates the importance of ensuring the availability of novel antibiotics.
Ethical approval
The study approval was taken from Institutional Ethics Committee, Institution Name: All India Institute of Medical Sciences, New Delhi, Approval Number: 207/11.04.2020, RP-47/2021, Date: 11.04.2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This work was supported by the Indian Council of Medical Research (ICMR), New Delhi, India (Project File No: 5/10/FR/56/2020-RBMCH).
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)–assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)–assisted technology for assistance in writing or editing of the manuscript, and no images were manipulated using AI.
References
- Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: A systematic review and meta-analysis. Lancet Infect Dis. 2020;1:1302-14.
- [CrossRef] [PubMed] [Google Scholar]
- High prevalence of Mycoplasma genitalium in men who have sex with men: A cross-sectional study. Indian J Dermatol Venereol Leprol. 2020;86:195.
- [CrossRef] [PubMed] [Google Scholar]
- Distribution study of Chlamydia trachomatis serovars among high-risk women in China performed using PCR-restriction fragment length polymorphism genotyping. J Clin Microbiol. 2007;45:1185-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Use of TaqMan 5′ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J Clin Microbiol. 2004;42:683-92.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Development of a rotor-gene real-time PCR assay for the detection and quantification of Mycoplasma genitalium. J Microbiol Methods. 2012;88:311-5.
- [CrossRef] [PubMed] [Google Scholar]
- Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Detection of markers predictive of macrolide and fluoroquinolone resistance in Mycoplasma genitalium from patients attending sexual health services in England. Sex Transm Infect. 2018;94:9-13.
- [CrossRef] [PubMed] [Google Scholar]
- Macrolide and fluoroquinolone resistance of Mycoplasma genitalium in southern Spain, 2018–2019. Sex Transm Infect. 2021;97:8-10.
- [CrossRef] [PubMed] [Google Scholar]
- Mycoplasma genitalium macrolide resistance update: Rate among a 2016–2017 cohort of patients in Barcelona, Spain. Enferm Infecc Microbiol Clin (Engl Ed). 2020;38:99-104.
- [CrossRef] [PubMed] [Google Scholar]
- High prevalence of circulating dual-class resistant Mycoplasma genitalium in asymptomatic MSM in Tokyo, Japan. JAC Antimicrob Resist. 2021;3:dlab091.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






