Translate this page into:
Clinical profile and photocontact sensitivity pattern in patients with cosmetic dermatitis: A prospective study
Corresponding author: Dr. Bijaylaxmi Sahoo, Department of Dermatology, Maulana Azad Medical College, New Delhi, India. blsahooacad@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Monalisa K, Sahoo B. Clinical profile and photocontact sensitivity pattern in patients with cosmetic dermatitis: A prospective study. Indian J Dermatol Venereol Leprol. 2024;90:479-85. doi: 10.25259/IJDVL_1110_2022
Abstract
Background
With the rise in cosmetic usage, adverse reactions related to cosmetics have also risen. Photocontact dermatitis to cosmetics is a challenging entity to diagnose and manage.
Objectives
To evaluate the clinical features and photocontact sensitivity patterns in patients with cosmetic dermatitis and establish their association based on patch and photopatch test results.
Methods
A prospective observational study, where 80 patients with a clinical diagnosis of cosmetic dermatitis were patch or photopatch tested (as per indication) with the Indian standard series, Indian cosmetic and fragrance series, and the patient’s personal product(s).
Results
A total of 104 positive reactions were observed in 57/80 patients, of which 50 were relevant to cosmetics usage. Sixty-five patients underwent a photopatch test, and 17 tested positive. Photosensitivity in patients was significantly associated with a positive photopatch test (p-value < 0.001). Various new photo-allergens were discovered, including propylene glycol, triethanolamine, chloroacetamide, isopropyl myristate, cetrimide and hexamine. Facial melanosis was a predominant clinical finding in 44 patients, with pigmented contact dermatitis detected in 19 (43.2%) of these cases.
Limitations
Patients’ personal products could not be tested on every patient. Chemical analysis of indigenous products and the individual chemical ingredients of the patient’s personal products could not be patch-tested separately. Phototesting was not performed in patients with photosensitivity.
Conclusion
In patients with suspected cosmetic dermatitis with history of photosensitivity or those with facial melanosis of unknown origin, a photopatch test is crucial to detect potentially hidden photo allergens. Many new photo allergens have emerged in the present study. Cosmetic companies should provide detailed information regarding each constituent of the cosmetic products.
Keywords
Allergic contact dermatitis
patch test
cosmetics
photosensitivity
facial melanosis
Introduction
Cosmetics include a wide range of personal care products.1 Pooled data from seven countries from North America and Europe found the prevalence of allergic contact dermatitis to cosmetics to be around 6.8% (2066/ 30,207).2 However, few studies have prospectively evaluated the photocontact sensitivity pattern to cosmetics in the Indian population. There is a need for each centre and country to develop its epidemiologic base.
The primary objective of this study was to know the frequency of positive patch and photopatch tests in patients with a clinical diagnosis of cosmetic dermatitis. The secondary objective was to analyse the cause and pattern of cosmetic dermatitis and understand its relevance.
Methods
This prospective observational study was conducted in the department of Dermatology, Maulana Azad Medical College and Lok Nayak Hospital, New Delhi, India from November 2017 to March 2019 following institutional ethical committee approval. Patients older than 18, with a clinical diagnosis of cosmetic dermatitis and sufficient understanding to give written informed consent, were included in this study.
Eighty patients were included in the study. The exclusion criteria included pregnant and lactating females, subjects applying topical corticosteroids over the back in the past 2 weeks or who were on systemic immunosuppressant in the past 4 weeks. Patient’s details were recorded (Supplementary appendix). Patch test was done using the Indian standard series and Indian cosmetic and fragrance series, Systopic India [Supplementary table S1,2]. The patient’s personal products, were tested in “as is” in 1% and 5% dilution.
Photopatch test was undertaken in patients with: i) exposed-site dermatitis, ii) exposed-site pruritus, or iii) photosensitivity after excluding other causes of photosensitivity. The allergens were applied over the back in duplication. At 48 hours, one set of allergens was covered with an opaque sheet, and the other was irradiated with UV-A at 10 J/cm2. The source of ultraviolet A was a phototherapy machine (VCare India UV therapy V2.0, 12 Philips TL 100 W/10 R of ultraviolet A and B each). The final reading was noted at 96 hours. Interpretation and Grading of the results were made by International Contact Dermatitis Research Group (IGDRG) criteria [Supplementary table S3]. The chemical composition of the cosmetics was noted [Supplementary table S4]. Relevance was determined as (i) Definite: If the Patch or use test with the suspected material is positive and re-exposure to the material causes recurrence of contact dermatitis, (ii) Probable: If the antigen is present in known skin contactants and the clinical presentation is consistent with that exposure, (iii) Possible: If skin contact with materials known to contain the allergen was likely, and (iv) Past: If the patch test is positive but the exposure was in the past, and not the present [Supplementary table S5]. Repeated open application and usage tests were done in high clinical suspicion, when the patch test results were not contributory. Patients were treated for symptomatic relief and were followed up for at least 6 weeks.
Statistical Analysis
The data was analysed using a statistical package for social sciences (SPSS) 17. The chi-square and Fisher exact tests were used to analyse the data. A p-value <0.05 was considered statistically significant.
Results
Baseline data
Of the 80 patients, there were 26 males and 54 females(age range: 20–49 years; mean age: 28.5 years). Among them, 34 had facial cosmetic dermatitis, 23 had hair dye dermatitis, 7 had Kumkum (vermilion) dermatitis, 5 had shaving cream or soap dermatitis, 3 had eye cosmetics dermatitis, and 2 had each lipstick and nail polish dermatitis. Additionally, 4 patients had contact dermatitis due to multiple cosmetic products.
Sixteen patients presented with acute, 27 with subacute, and 37 with chronic dermatitis. The duration of dermatitis varied. Most patients (41) had dermatitis for 1–5 years, followed by 25 patients who had it for less than a month. Most patients (61) experienced dermatitis within 24 hours of exposure. Atopy was found in 5 patients. Regarding occupation, the majority of patients (34) were involved in household work, followed by professionals (17).
Patch test data
Of 80 patients; 57 with allergic contact dermatitis (81 allergic reactions) and 23 were with Irritant contact dermatitis [Table 1]. In addition, 25 patients also underwent testing with their personal products with 17 different products; 23 (92%) patients revealed 31 positive reactions [Table 2]. Repeated open application tests were done in 5 patients, and one with lipstick dermatitis revealed a positive result at the end of 1 week.
Photopatch test data
Sixty-five patients underwent a photopatch test; 17 (26.1%) patients revealed 18 photoallergic and 5 photo augmented reactions. Photosensitivity was found in 16 patients, who revealed positive photopatch test reactions to thiomersal, kathon CG, chloroacetamide, fragrance mix, cetrimide, chlorhexidine, triethanolamine, propylene glycol, isopropyl myristate, hexamine, and nickel [Figures 1 and 2]. We found the association of photosensitivity to be statistically significant (P-value < 0.001, 95% C.I: 0.8227 to 0.9813) in patients with photo-contact dermatitis compared to patients with lone allergic contact dermatitis to cosmetics.
Pigmented contact dermatitis [Figures 3 and 4] was an additional finding observed in 44 patients, where 19 (43.2%) patients revealed clinically relevant reactions. This included photoallergic reactions to fragrance mix, cetrimide and hexamine and allergic reactions to p-phenylenediamine, nickel, lavender absolute, triethanolamine, chloroacetamide, benzyl salicylate, diazolidinyl urea, musk mix, geranium oil, rose oil, and colophony.
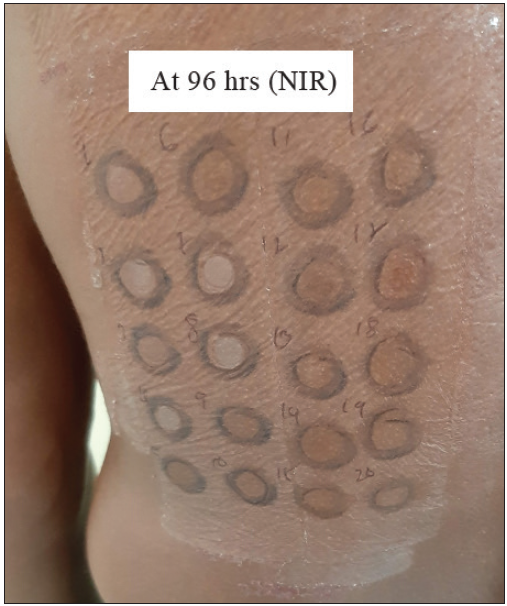
- Photoallergic reaction to fragrance mix (FM) at 96 hours. The non-irradiated (NIR) side shows an adverse reaction with FM (No. 17).
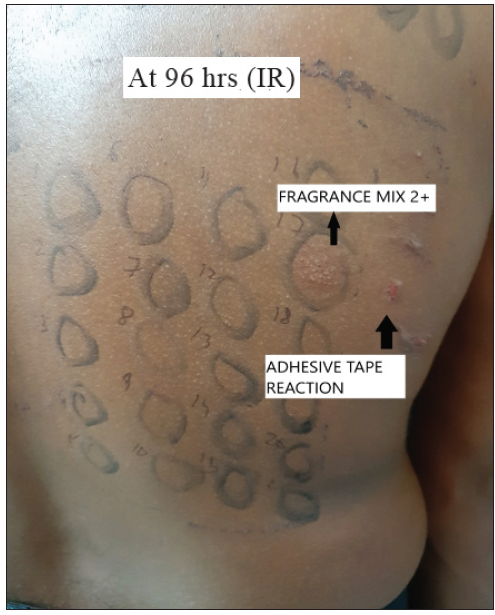
- Irradiated side (IR) showing a strongly positive reaction (2+) with FM (No. 17) and adhesive tape reaction.
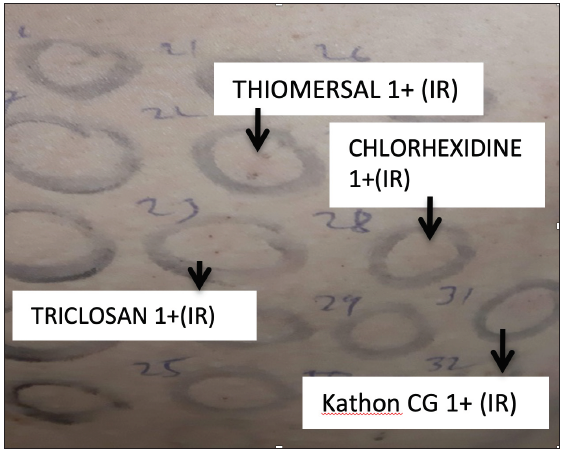
- Photoallergic mild (1+) reaction to Kathon CG, thiomersal, chlorhexidine and triclosan in a patient with contact dermatitis to facial cosmetics.
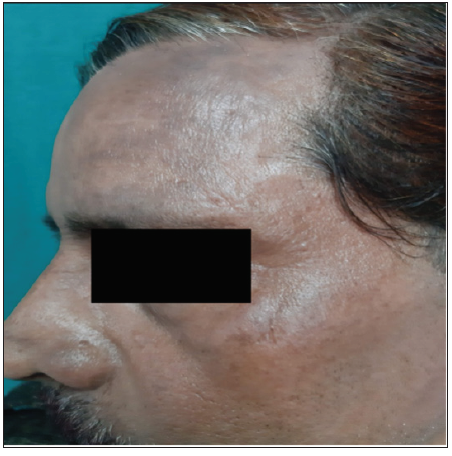
- Hyperpigmented macules present over the scalp hairline and forehead region in patients with allergic contact dermatitis to hair dye.
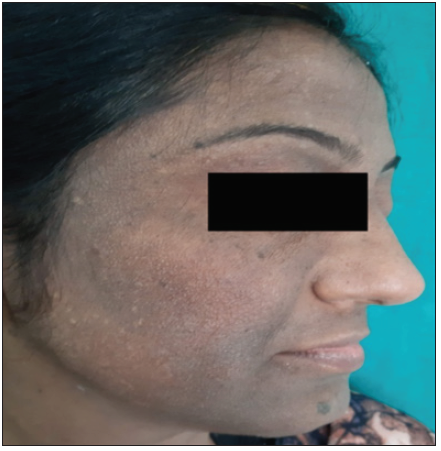
- Diffuse hyperpigmentation over the face predominantly involving the forehead, cheeks and chin in patients with contact dermatitis to facial cosmetics.
| Allergens | Allergic, n | Photoallergic, n | Photoaugmented, n | Total, n | Relevant reactions‡, n |
|---|---|---|---|---|---|
| 1. PRESERVATIVES | |||||
| Thiomersal† (0.1% Pet.) | 19 | 2 | 2 | 23 | 0 |
| Paraben mix (15% Pet.) | 1 | - | - | 1 | 1 |
| Kathon CG† (1.3% Aq.) | - | 1 | - | 1 | 0 |
| Formaldehyde (1.1% Aq.) | 1 | - | - | 1 | 0 |
| Diazolidinyl urea (2% Aq.) | - | - | 2 | 2 | 1 |
| Chlorocresol (1% Pet.) | 1 | - | - | 1 | 0 |
| Chloroacetamide† (0.2% Pet.) | 1 | 1 | - | 2 | 1 |
| Total | 23 | 4 | 4 | 31 | 3 |
| 2. HAIR DYE COMPONENT | |||||
| p-phenylenediamine (1% Pet.) | 19 | - | 1 | 20 | 19 |
| 3. FRAGRANCES | |||||
| Balsam of Peru (25% Pet.) | 2 | - | - | 2 | 1 |
| Musk mix (5% Pet.) | 3 | - | - | 3 | 2 |
| Lavender absolute (2% Pet.) | 1 | - | - | 1 | 1 |
| Benzyl salicylate (2% Pet.) | 1 | - | - | 1 | 1 |
| Fragrance mix† (8% Pet.) | 5 | 1 | - | 6 | 5 |
| Geranium oil (2% Pet.) | 1 | - | - | 1 | 1 |
| Rose oil (2% Pet.) | 1 | - | - | 1 | 1 |
| Total | 14 | 1 | - | 15 | 12 |
| 4. ANTISEPTICS AND DISINFECTANT | |||||
| Cetrimide† (0.5% Pet.) | 9 | 3 | - | 12 | 6 |
| Chlorhexidine† (0.5% Aq.) | - | 2 | - | 2 | 0 |
| Total | 9 | 5 | - | 14 | 6 |
| 5. RESIN | |||||
| Colophony (1% Pet.) | 2 | - | - | 2 | 1 |
| Epoxy resin (1% Pet.) | 3 | - | - | 3 | 1 |
| Total | 5 | - | - | 5 | 2 |
| 6. VEHICLE | |||||
| Triethanolamine† (2% Pet.) | 1 | 1 | - | 2 | 1 |
| Propylene glycol† (5% Aq.) | - | 1 | - | 1 | 1 |
| Isopropyl myristate† (20% Pet.) | - | 1 | - | 1 | 0 |
| Total | 1 | 3 | - | 4 | 2 |
| 7. OTHERS | |||||
| Mercaptobenzothiazole (2% Pet.) | 1 | - | - | 1 | 1 |
| Black rubber mix (0.6% Pet.) | 1 | - | - | 1 | 0 |
| Hexamine† (2% Pet.) | 2 | 3 | - | 5 | 1 |
| Nickel† (5% Pet.) | 1 | 2 | - | 3 | 3 |
| Sorbitan sesquilate (SSQ) (2% Pet.) | 1 | - | - | 1 | 0 |
| Triclosan (2% Pet.) | 1 | - | - | 1 | 0 |
| Benzotriazole (1% Pet.) | 1 | - | - | 1 | 1 |
| Butylated hydroxytoluene (2% Pet.) | 1 | - | - | 1 | 0 |
| Tween 80 (1% Pet.) | 1 | - | - | 1 | 0 |
| Total | 10 | 5 | - | 15 | 6 |
| Total Allergens Positive At 96 Hours | 81 (77.9) | 18 (17.3) | 5 (4.8) | 104 | 50 |
n, absolute numbers. Aq., aqueous. Pet., petrolatum.
| Personal products* | Allergic, n | Photoallergic, n | Photoaugmented, n | Total, n | Repeated open application test |
|---|---|---|---|---|---|
| Neha Mehandi | 3 | - | - | 3 | - |
| Garnier Hair Color | 8 | - | 1 | 9 | - |
| Godrej Hair Dye | 9 | - | - | 9 | - |
| Ponds Cold Cream | - | 1 | - | 1 | - |
| Lifebuoy soap | - | - | - | - | Negative |
| Melas cream | - | - | - | - | Negative |
| Fair and Lovely cream | - | - | - | - | Negative |
| Zariba lipstick | - | - | - | - | Positive on the 7th day |
| Ayur Body Lotion | - | - | - | - | The patient lost to follow up for the reading. |
| Himalaya Body Lotion | - | - | - | - | Negative |
| Kumkum (indigenous), company NK | 3 | 1 | - | 4 | - |
| Eyeliner (Company NK) | 1 | - | - | 1 | - |
| Huda Beauty Liquid Matte lipstick | 1 | - | - | 1 | - |
| Oriflame Pure Colour lipstick | 1 | - | - | 1 | - |
| ADS Sindoor | 1 | - | - | 1 | - |
| Keo Karpin Hair Oil | 1 | - | - | 1 | - |
| Total | 28 | 2 | 1 | 31 |
n, absolute numbers. Few patients tested positive for more than one personal products
Preservatives (n = 31, 29.8%), hair dye (n = 20, 19.2%) and fragrance (n = 15, 14.4%) were the predominant contributors to patch or photopatch test reactions [Table 1]. The top three reactions identified were to thiomersal (n = 23, 22.1%), p-phenylenediamine (n = 20, 19.2%) and cetrimide (n = 12, 11.5%) [Table 1] 50 (48.1%) reactions were found relevant to the patient’s current cosmetic usage (reactions with definite and probable relevance were considered clinically relevant) [Table 1].
The details of the allergens and photo allergens among the various cosmetics group are summarised in Table 3 and Supplementary tables 6, 7, and 8.
| Allergens | Allergic, n | Photoallergic, n | Photoaugmented, n | Total, n | Relevance, n |
|---|---|---|---|---|---|
| 1. Hair dye | |||||
| Thiomersal (0.1% Pet.) | 2 | 1 | 1 | 4 | 0 |
| p-phenylenediamine (1% Pet.) | 13 | - | 1 | 14 | 14 |
| Balsam of Peru (25% Pet.) | 1 | - | - | 1 | 0 |
| Colophony (1% Pet.) | 1 | - | - | 1 | 0 |
| Fragrance mix (8% Pet.) | 1 | - | - | 1 | 1 |
| Mercaptobenzothiazole (2% Pet.) | 1 | - | - | 1 | 1 |
| Black rubber mix (0.6% Pet.) | 1 | - | - | 1 | 0 |
| Propylene glycol (5% Aq.) | - | 1 | - | 1 | 0 |
| Epoxy resin (1% Pet.) | 2 | - | - | 2 | 0 |
| Hexamine (2% Pet.) | - | 1 | - | 1 | 0 |
| Chlorhexidine (0.5% Aq.) | - | 1 | - | 1 | 0 |
| Kathon CG (1.3% Aq.) | - | 1 | - | 1 | 0 |
| Nickel (5% Pet.) | - | 1 | - | 1 | 1 |
| Musk mix (5% Pet.) | 1 | - | - | 1 | 0 |
| Sorbitan sesquilate (2% Pet.) | 1 | - | - | 1 | 0 |
| Triclosan (2% Pet.) | 1 | - | - | 1 | 0 |
| Cetrimide (0.5% Pet.) | 1 | - | - | 1 | 0 |
| Total allergens positive reactions at 96 hrs | 26 | 6 | 2 | 34 | 17 |
| 2. Facial cosmetic | |||||
| Thiomersal (0.1% Pet.) | 14 | 1 | 1 | 16 | 0 |
| Cetrimide (0.5% Pet.) | 6 | 2 | - | 8 | 5 |
| Fragrance mix (8% Pet.) | - | 1 | - | 1 | 1 |
| Lavender absolute (2% Pet.) | 1 | - | - | 1 | 1 |
| Hexamine (2% Pet.) | 1 | 1 | - | 2 | 0 |
| Chlorhexidine (0.5% Aq.) | - | 1 | - | 1 | 0 |
| Formaldehyde (1.1% Aq.) | 1 | - | - | 1 | 0 |
| Benzyl salicylate (2% Pet.) | 1 | - | - | 1 | 1 |
| Chloroacetamide (0.2% Pet.) | - | 1 | - | 1 | 1 |
| Triethanolamine (2% Pet.) | - | 1 | - | 1 | 1 |
| Benzotriazole (1% Pet.) | 1 | - | - | 1 | 1 |
| PPD (1% Pet.) | 1 | - | - | 1 | 0 |
| Diazolidinyl urea (2% Aq.) | - | - | 2 | 2 | 1 |
| Isopropyl myristate (20% Pet.) | - | 1 | - | 1 | 0 |
| Total allergens positive reactions at 96 hrs | 26 | 9 | 3 | 38 | 12 |
| Kumkum | |||||
| p-phenylenediamine (1% Pet.) | 3 | - | - | 3 | 3 |
| Hexamine (2% Pet.) | 1 | - | - | 1 | 0 |
| Butylated hydroxytoluene (2% Pet.) | 1 | - | - | 1 | 0 |
| Chlorocresol (1% Pet.) | 1 | - | - | 1 | 0 |
| Thiomersal (0.1% Pet.) | 2 | - | - | 2 | 0 |
| Fragrance mix (8% Pet.) | 1 | - | - | 1 | 1 |
| Triethanolamine (2% Pet.) | 1 | - | - | 1 | 0 |
| Nickel (5% Pet.) | - | 1 | - | 1 | 1 |
| Chloroacetamide (0.2% Pet.) | 1 | - | - | 1 | 0 |
| Tween 80 (1% Pet.) | 1 | - | - | 1 | 0 |
| Geranium oil (2% Pet.) | 1 | - | - | 1 | 1 |
| Rose oil (2% Pet.) | 1 | - | - | 1 | 1 |
| Musk mix (5% Pet.) | 1 | - | - | 1 | 1 |
| Cetrimide (0.5% Pet.) | 1 | 1 | - | 2 | 0 |
| Total allergens positive at 96 hrs (IR) | 16 | 2 | - | 18 | 8 |
| Eyeliner | |||||
| Hexamine (2% Pet.) | - | 1 | - | 1 | 1 |
| Nickel (5% Pet.) | 1 | - | - | 1 | 1 |
| Total allergens positive at 96 hrs | 1 | - | 1 | 2 | 2 |
| Lipstick | |||||
| ROAT- positive on 7th day | |||||
| Shaving cream | |||||
| Cetrimide (0.5% Pet.) | 1 | - | - | 1 | 1 |
| Thiomersal (0.1% Pet.) | 1 | - | - | 1 | 0 |
| Total allergens positive at 96 hrs | 2 | - | - | 2 | 1 |
| Multiple cosmetics | |||||
| p-phenylenediamine (1% Pet.) | 2 | - | - | 2 | 2 |
| Fragrance mix (8% Pet.) | 3 | - | - | 3 | 2 |
| Musk mix (5% Pet.) | 1 | - | - | 1 | 1 |
| Colophony (1% Pet.) | 1 | - | - | 1 | 1 |
| Epoxy resin (1% Pet.) | 1 | - | - | 1 | 1 |
| Paraben mix | 1 | - | - | 1 | 1 |
| Balsam of Peru (25% Pet.) | 1 | - | - | 1 | 1 |
| Total allergens positive at 96 hrs | 10 | - | - | 10 | 10 |
n: absolute numbers; Aq.: aqueous; Pet.: petrolatum; PPD: paraphenylenediamine; ROAT: repeated open application test.
Patients were advised to refrain from the concerned personal care products and informed about the risk of cross-reactivity to other related chemicals. None of the patients relapsed within 6 weeks of follow-up.
Discussion
With the increasing use of cosmetics, there has been a corresponding rise in adverse reactions related to these products. The present study found that 71.2% (n = 57) of patients exhibited a positive patch test for cosmetics. This frequency is comparable to previous studies reported by Penchalaiah et al. (68%) in North India and Mayo Clinic Contact Dermatitis Group (68.4%) in the USA.3,4 This indicates a significant prevalence of positive patch test reactions among individuals using cosmetics.
Another concerning finding was the occurrence of photoallergic and photoaugmented reactions in 26.1% (n = 17) cases. Compared to a previous study from the same institution,5 there has been a significant rise (2-fold) in the frequency of photoallergic reactions over the past two decades.
In the present study, we observed the occurrence of pigmented contact dermatitis (43.2%) in a considerable subset of cases. Prior investigations have explored its association with various allergens.6–9 However, our study takes a step further by shedding light on the connection between pigmented contact dermatitis and various photo allergens.
Interestingly, the study observed multiple photoallergic and photoaugmented reactions. It is well known that photoallergy is a combined immunological reaction, where the action spectrum generally shifts to a longer wavelength.10 In the present study, among the various photoallergens detected, nickel, fragrance mix, propylene glycol, triethanolamine, chloroacetamide, cetrimide, and hexamine were found in the patients’ cosmetic products, indicating a direct exposure. Past exposure to the remaining allergens was inferred from the patients’ history.
A retrospective study conducted by Hu et al. revealed that photoallergic reactions to thiomersal, nickel, and chlorhexidine were observed in 9.8% (n = 553), 2.6% (n = 143), and 1.5% (n = 20) of photopatch test reactions, respectively.11 Additionally, anecdotal cases have been reported illustrating the photoallergic reactions to fragrance mix and photoaugmented reactions to kathon CG, thiomersal and p-phenylenediamine.12–16 However, the photoallergic nature of propylene glycol, triethanolamine, chloroacetamide, isopropyl myristate, cetrimide, kathon CG, hexamine and photoaugmented reactions to diazolidinyl urea, has not been noted earlier.
We also observed that the manufacturers did not provide complete information on the cosmetic ingredients, thus making it difficult to ascertain the presence of various hidden chemicals in the cosmetic products.
Limitations
Patients’ personal products could not be tested in every patient. Chemical analysis of indigenous products and testing with individual chemical ingredients of the patient’s personal products could not be patch-tested. Phototesting was not done in patients with photosensitivity.
Conclusion
This study highlights a significant prevalence of cosmetic dermatitis (71.2%) among the Indian population. Moreover, an alarming rise in photoallergic and photoaugmented reactions (26.1%) is evident. The coexistent pigmented contact dermatitis in a considerable number of cases emphasises its potential link with cosmetics ingredients. The study emphasises the need for comprehensive cosmetic labelling and increased awareness among manufacturers and consumers to ensure safety and prevent adverse reactions.
Ethical approval
The Institutional Review Board approval was obtained.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- A primer on cosmetics. AAD advisory board, CTFA task force on cosmetics. J Am Acad Dermatol. 1992;27:469-84.
- [CrossRef] [PubMed] [Google Scholar]
- Allergic contact dermatitis to cosmetics. Dermatol Clin. 2006;24:215-32.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitizers commonly causing allergic contact dermatitis from cosmetics. Contact Dermatitis. 2000;43:311-3.
- [PubMed] [Google Scholar]
- Results of patch testing to personal care product allergens in a standard series and a supplementary cosmetic series: An analysis of 945 patients from the Mayo Clinic Contact Dermatitis Group, 2000–2007. J Am Acad Dermatol. 2010;63:789-98.
- [CrossRef] [PubMed] [Google Scholar]
- Cosmetic dermatitis - Current perspectives. Int J Dermatol. 2003;42:533-42.
- [CrossRef] [PubMed] [Google Scholar]
- Patch testing and histopathology in Thai patients with hyperpigmentation due to erythema dyschromicum perstans, Lichen planus pigmentosus, and pigmented contact dermatitis. Asian Pac J Allergy Immunol. 2014;32:185-92.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical profile and allergens in pigmented cosmetic dermatitis and allergic contact dermatitis to cosmetics in India. Dermatitis. 2018;29:1.
- [Google Scholar]
- The role of patch testing with indian cosmetic series in patients with facial pigmented contact dermatitis in India. Indian J Dermatol. 2021;66:81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Patch testing as a corroborative and diagnostic tool in patients suspected of contact allergen induced facial melanosis. Indian J Dermatol. 2021;66:337-42.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Photopatch testing in Chinese patients over 10 years. Dermatitis. 2016;27:137-42.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of photopatch test allergens for Indian patients of photodermatitis: Preliminary results. Indian J Dermatol Venereol Leprol. 2011;77:148.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of photopatch test series in India. Contact Dermatitis. 2007;56:168-9.
- [CrossRef] [PubMed] [Google Scholar]
- Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-5.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of the results from the patch test to para-phenylenediamine in the TRUE test in patients with a hair dye contact allergy. Ann Dermatol. 2015;27:171-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Hair dye dermatitis and para-phenylenediamine contact sensitivity. Indian Dermatol Online J. 2015;6:246-7.
- [PubMed] [PubMed Central] [Google Scholar]






