Translate this page into:
Dermatoscopic evaluation of leprosy: A multi-centre cross-sectional study
Corresponding Author: Dr. Keshavamurthy Vinay, Department of Dermatology, Venereology, and Leprology and Postgraduate Institute of Medical Education and Research, Chandigarh, India. vinay.keshavmurthy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ankad B, Sharma A, Vinay K, Rathod S, Mehta H, Bhat YJ, et al. Dermatoscopic evaluation of leprosy: A multi-centre cross-sectional study. Indian J Dermatol Venereol Leprol. 2024;90:486-93. doi: 10.25259/IJDVL_506_2023
Abstract
Background
Leprosy is known to be a great mimicker. Its dermatoscopic evaluation may be of value in establishing diagnosis.
Objective
To study the dermatoscopic findings encountered across the leprosy spectrum.
Methods
This was a multi-centre cross-sectional study of leprosy patients, where after a thorough cutaneous and neurological evaluation, representative skin lesions from the study patients were photographed in standard settings, and the most representative skin lesions were chosen for dermatoscopic evaluation. Data was entered in a structured proforma and a descriptive analysis of dermatoscopic patterns was carried out.
Results
A total of 53 cases of ages between 14 and 80 years, ranging from tuberculoid to the lepromatous spectrum of disease, with active skin lesions in the form of patches and plaques were included. The spectrum of leprosy as per Ridley-Jopling classification at diagnosis was indeterminate in 1 (1.9%), tuberculoid in 1 (1.9%), borderline tuberculoid in 25 (21.5%), borderline lepromatous in 9 (17%), lepromatous in 14 (26.4%) and histoid in 3 (5.7%). Dermatoscopic features included distorted pigment network in 48 (90.6%), focal white areas in 40 (75.5%), reduced eccrine and follicular openings in 43 (81.1%), widened skin lines in 28 (52.8%), circle hairs in 15 (28.3%) and white shiny streaks in 17 (32.1%).
Conclusion
Dermatoscopy is a practical, non-invasive device to assess skin lesions of leprosy and provide cues to its diagnosis, spectral classification and differentiating it from other granulomatous disorders. However, dermatoscopy alone cannot reliably differentiate between the various types of leprosy and future large-scale studies are required.
Limitations of the study
The numbers for each subtype were variable and few in some spectrum of leprosy patients. A dermatoscopic-histologic correlation was not done.
Keywords
Dermatoscopy
granulomatous disease
leprosy
skin of colour
Introduction
Leprosy is a chronic granulomatous infectious disease caused by M. leprae and M. lepromatosis, and its clinical presentations exhibit widely varying morphological characteristics. Dermatoscopy is a non-invasive examination tool which can help in diagnosis and categorising leprosy into various sub-groups.1
Methodology
This multi-centre cross-sectional study of leprosy patients was conducted from November 2021 to April 2022. After a thorough cutaneous and neurological evaluation followed by histopathological confirmation of diagnosis, representative skin lesions from the study patients were photographed and the most representative skin lesions were chosen for dermatoscopic evaluation.
Dermatoscopic evaluation was performed using a hand-held DL4 DermLite dermatoscope at 10x magnification in polarised contact mode, and photographs were captured and archived. The archived dermatoscopic images were analysed by the authors BA and KV for a set of pre-decided dermatoscopic features as listed in Table 1. Discrepancy in opinions, if any, were sorted by personal discussions. Any new dermatoscopic features observed were also documented. The histopathological specimen was obtained prior to treatment initiation from the most downward leprosy spectrum as per the standard practice and did not always correlate with the dermatoscopic lesion. Skin biopsy was also repeated at the end of treatment as part of the standard of care.
| Dermatoscopic finding | Percentage of patients exhibiting the finding n = 53 |
|---|---|
| Distorted pigment network | 48 (90.6%) |
| Focal white areas | 40 (75.5%) |
| Reduced follicular and eccrine openings | 43 (81.1%) |
| Widened skin lines | 28 (52.8%) |
| Brownish background | 24 (45.3%) |
| Linear vessels | 26 (49.0%) |
| Branching vessels | 16 (30.2%) |
| Milky red globules | 16 (30.2%) |
| Dotted vessels | 2 (3.8%) |
| Pinkish hue | 22 (41.5%) |
| Circle hairs | 15 (28.3%) |
| Broken hairs | 17 (32.1%) |
| White rosettes | 2 (3.8%) |
| Follicular plugs | 3 (5.7%) |
| White shiny streaks | 17 (32.1%) |
| Whitish-yellow globules | 27 (50.9%) |
| Scales | 16 (30.2%) |
| Bluish area due to clofazimine | 2 (3.8%) |
| Serocrusts | 1 (1.9%) |
Results
A total of 53 patients aged between 14 and 80 years were included. Of these, there were 33 (62.3%) males and 20 (37.7%) females. The median duration of symptoms at presentation was 10.5 months (1–120 month range). The spectrum of leprosy as per Ridley–Jopling classification at diagnosis was indeterminate in 1 (1.9%), tuberculoid (TT) in 1 (1.9%), borderline tuberculoid (BT) in 25 (21.5%), borderline lepromatous (BL) in 9 (17%), lepromatous (LL) in 14 (26.4%) and histoid in 3 (5.7%). There were no defaulters and only one relapse case. Reactions were seen only in 9 (17%) patients, of which 4 (7.5%) were type 1 reactions (T1R) in BT spectrum and 5 (9.4%) were type 2 reactions (T2R) in LL spectrum. Slit skin smear (SSS) was positive in 38 (71.7%) patients. The mean bacteriological index (BI) at baseline was 2.02 ± 1.86. Fifteen out of 38 patients had a BI > 1. The majority of the patients received the World Health Organization (WHO) multi-drug therapy (MDT) multibacillary regimen (WHO MDT-MBR). Of the 53 patients, 19 (35.8%) had type IV, 31 (58.5%) had type V and 3 (5.7%) had type VI Fitzpatrick skin phototype.
Dermatoscopic evaluation of all patients was done, the results of which are summarised in Table 1 and Figures 1, 2 and 3. Brownish background was fairly well represented in non-reactive lesions [Figure 1]. The various dermatoscopic features found were widened skin lines [Figures 1a, 1b and 1c], focal white areas [Figures 1b, 1c and 1d] and distorted pigment networks [Figures 1a–1d and 2c]. Other features included and circle hairs [Figures 1a, 1c and 2b], follicular plugs [Figures 2a and 3a] and white rosettes [Figure 3b]. Only two patients showed bluish pigmentation due to clofazimine [Figure 2d] Vascular changes consisted of linear [Figures 1d, 3a and 3b], branching [Figures 1d, 3a and 3b], globular and linear blurry vessels [Figure 2a]. Whitish-yellow globules [Figure 1d] and white shiny streaks [Figures 2c and 3a] were also noted.
![Dermatoscopy of tuberculoid leprosy shows widened skin lines (arrow), distorted pigment network (box) and broken hairs (appear as black dots) (circles) over a brownish background. [Inset: respective clinical image showing a hypopigmented xerotic plaque with loss of appendages]](/content/126/2024/90/4/img/IJDVL-90-4-486-g1.png)
- Dermatoscopy of tuberculoid leprosy shows widened skin lines (arrow), distorted pigment network (box) and broken hairs (appear as black dots) (circles) over a brownish background. [Inset: respective clinical image showing a hypopigmented xerotic plaque with loss of appendages]
![Dermatoscopy of borderline tuberculoid leprosy shows focal white areas (star), widened skin lines (arrow) and distorted pigment network (circle) over a brownish background. [Inset: respective clinical image]](/content/126/2024/90/4/img/IJDVL-90-4-486-g2.png)
- Dermatoscopy of borderline tuberculoid leprosy shows focal white areas (star), widened skin lines (arrow) and distorted pigment network (circle) over a brownish background. [Inset: respective clinical image]
![Dermatoscopy of borderline lepromatous leprosy shows focal white areas (stars), widened skin lines (arrow) and distorted pigment network (circle). Broken (diamond) and circle (box) hairs are well appreciated. [Inset: respective clinical image]](/content/126/2024/90/4/img/IJDVL-90-4-486-g3.png)
- Dermatoscopy of borderline lepromatous leprosy shows focal white areas (stars), widened skin lines (arrow) and distorted pigment network (circle). Broken (diamond) and circle (box) hairs are well appreciated. [Inset: respective clinical image]
![Dermatoscopy of lepromatous leprosy shows focal white areas (stars), yellowish-white globules (hexagons) and linear (arrow) and branching (diamond) vessels. Distorted pigment network (circle) is well appreciated. [Inset: respective clinical image]](/content/126/2024/90/4/img/IJDVL-90-4-486-g4.png)
- Dermatoscopy of lepromatous leprosy shows focal white areas (stars), yellowish-white globules (hexagons) and linear (arrow) and branching (diamond) vessels. Distorted pigment network (circle) is well appreciated. [Inset: respective clinical image]
![Dermatoscopy of type 1 reaction shows short liner blurry vessels (circle), follicular plugs (arrows) and scales (box) over a pinkish background. [Inset: respective clinical image]](/content/126/2024/90/4/img/IJDVL-90-4-486-g5.png)
- Dermatoscopy of type 1 reaction shows short liner blurry vessels (circle), follicular plugs (arrows) and scales (box) over a pinkish background. [Inset: respective clinical image]
![Dermatoscopy of type 2 reaction shows perifollicular scales (arrows) and circle hairs (circle) over a pinkish background (star). [Inset: respective clinical image]](/content/126/2024/90/4/img/IJDVL-90-4-486-g6.png)
- Dermatoscopy of type 2 reaction shows perifollicular scales (arrows) and circle hairs (circle) over a pinkish background (star). [Inset: respective clinical image]
![Dermatoscopy of borderline lepromatous leprosy shows white shiny streaks (arrow), distorted pigment network (circle) and pinkish hue (star). [Inset: respective clinical image]](/content/126/2024/90/4/img/IJDVL-90-4-486-g7.png)
- Dermatoscopy of borderline lepromatous leprosy shows white shiny streaks (arrow), distorted pigment network (circle) and pinkish hue (star). [Inset: respective clinical image]
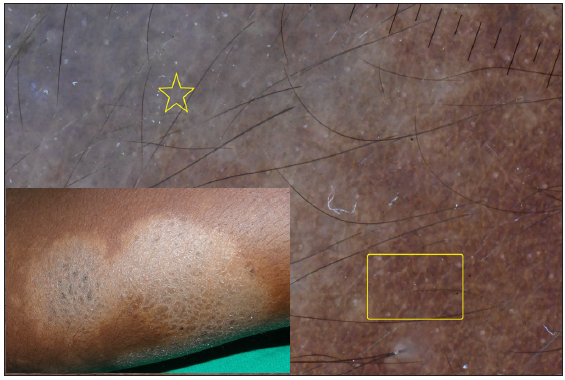
- Dermatoscopy of borderline tuberculoid leprosy showing bluish pigmentation (yellow star) and distorted pigment network (yellow box).
![Dermatoscopy of histoid leprosy showing follicular plugging (stars), white shiny streaks (arrow) and pinkish background. Note the linear (box) and branching vessels (circle). [Inset: respective clinical image]](/content/126/2024/90/4/img/IJDVL-90-4-486-g9.png)
- Dermatoscopy of histoid leprosy showing follicular plugging (stars), white shiny streaks (arrow) and pinkish background. Note the linear (box) and branching vessels (circle). [Inset: respective clinical image]
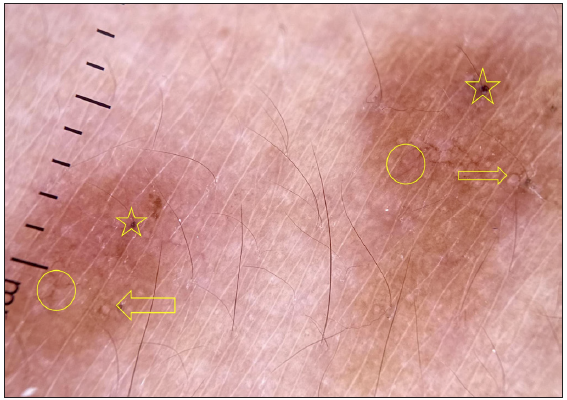
- Dermatoscopy of histoid leprosy where follicular plugs (yellow stars), white rosettes (yellow arrow) and branching vessels (yellow circle) with distorted pigment network are well appreciated.
Dermatoscopic features based on BI
There was no statistically significant difference in the dermatoscopic features across the spectrum (p > 0.05) [Table 2]. In the tuberculoid spectrum with BI 0/1, pigmentary and follicular changes were seen more commonly, whereas vascular changes and whitish-yellow globules were prominent in lesions with BI > 1.
|
Bacteriological index: BI = 0/1 (n-16) |
DPN | FWA | RFEO | WSL | BB | CH | BH | WR | FP | LV | BV | MBG | DV | PH | WSS | WYG | Sc | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Ind (n = 1) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
||||||||||||||||||||||||||
|
TT (n = 1) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
||||||||||||||||||||||
|
BT (n = 7) |
6 (85.7%) |
7 (100%) |
5 (71.4%) |
3 (42.9%) |
2 (28.6%) |
1 (14.3%) |
3 (42.9%) |
5 (71.4%) |
5 (71.4%) |
3 (42.9%) |
6 (85.7%) |
6 (85.7%) |
3 (42.9%) |
||||||||||||||||||
|
BL (n = 4) |
4 (100%) |
4 (100%) |
4 (100%) |
4 (100%) |
3 (75%) |
1 (25%) |
2 (50%) |
1 (25%) |
1 (25%) |
2 (50%) |
3 (75%) |
2 (50%) |
1 (25%) |
||||||||||||||||||
|
LL (n = 3) |
1 (33.3%) |
1 (33.3%) |
1 (33.3%) |
1 (33.3%) |
1 (33.3%) |
1 (33.3%) |
1 (33.3%) |
1 (33.3%) |
1 (33.3%) |
||||||||||||||||||||||
|
T2R (n = 2) |
2 (100%) |
2 (100%) |
2 (100%) |
1 (50%) |
1 (50%) |
1 (50%) |
1 (50%) |
1 (50%) |
2 (100%) |
2 (100%) |
1 (50%) |
1 (50%) |
|||||||||||||||||||
|
Bacteriological index: BI => 1 (n-37) |
DPN | FWA | RFEO | WSL | BB | CH | BH | WR | FP | LV | BV | MBG | DV | PH | WSS | WYG | Sc | ||||||||||||||
|
BT (n = 18) |
14 (77.8%) |
13 (72.2%) |
15 (83.3%) |
12 (66.7%) |
8 (44.4%) |
8 (44.4%) |
2 (11.1%) |
3 (16.7%) |
2 (11.1%) |
1 (5.6%) |
1 (5.6%) |
2 (11.1%) |
3 (16.7%) |
||||||||||||||||||
|
BL (n = 5) |
4 (80%) |
4 (80%) |
3 (60%) |
2 (40%) |
1 (20%) |
2 (40%) |
1 (20%) |
2 (40%) |
2 (40%) |
2 (40%) |
2 (40%) |
2 (40%) |
1 (20%) |
2 (40%) |
|||||||||||||||||
|
LL (n = 11) |
5 (45.5%) |
3 (27.3%) |
2 (18.2%) |
1 (9.1%) |
3 (27.3%) |
1 (9.1%) |
5 (45.5%) |
2 (18.2%) |
1 (9.1%) |
3 (27.3%) |
7 (63.6%) |
5 (45.5%) |
1 (9.1%) |
||||||||||||||||||
|
HL (n = 3) |
3 (100%) |
2 (66.7%) |
3 (100%) |
1 (33.3%) |
2 (66.7%) |
2 (66.7%) |
1 (33.3%) |
3 (100%) |
3 (100%) |
1 (33.3%) |
3 (100%) |
2 (66.7%) |
3 (100%) |
||||||||||||||||||
|
T1R (n = 4) |
4 (100%) |
2 (50%) |
4 (100%) |
2 (50%) |
2 (50%) |
2 (50%) |
4 (100%) |
1 (25%) |
3 (75%) |
1 (25%) |
3 (75%) |
1 (25%) |
2 (50%) |
3 (75%) |
|||||||||||||||||
|
T2R (n = 3) |
3 (100%) |
2 (66.7%) |
2 (66.7%) |
2 (66.7%) |
2 (66.7%) |
1 (33.3%) |
1 (33.3%) |
1 (33.3%) |
1 (33.3%) |
3 (100%) |
1 (33.3%) |
||||||||||||||||||||
| Disease duration: < 6 months (n-23) | DPN | FWA | RFEO | WSL | BB | CH | BH | WR | FP | LV | BV | MBG | DV | PH | WSS | WYG | Sc | ||||||||||||||
|
BT (n = 13) |
11 (84.6%) |
10 (76.9%) |
11 (84.6%) |
9 (69.2%) |
5 (38.5%) |
6 (46.2%) |
1 (7.7%) |
5 (38.5%) |
2 (15.4%) |
3 (23.1%) |
1 (7.7%) |
3 (23.1%) |
3 (23.1%) |
2 (15.3%) |
|||||||||||||||||
|
BL (n = 2) |
2 (100%) |
2 (100%) |
1 (50%) |
1 (50%) |
2 (100%) |
1 (50%) |
1 (50%) |
2 (100%) |
1 (50%) |
1 (50%) |
1 (50%) |
||||||||||||||||||||
|
LL (n = 6) |
2 (33.3%) |
1 (16.7%) |
1 (16.7%) |
4 (66.7%) |
2 (33.3%) |
1 (16.7%) |
5 (83.3%) |
3 (50%) |
|||||||||||||||||||||||
|
HL (n = 2) |
1 (50%) |
1 (50%) |
2 (100%) |
1 (50%) |
1 (50%) |
1 (50%) |
1 (50%) |
2 (100%) |
2 (100%) |
1 (50%) |
2 (100%) |
2 (100%) |
2 (100%) |
||||||||||||||||||
|
T1R (n = 1) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
|||||||||||||||||||||||
|
T2R (n = 2) |
2 (100%) |
1 (50%) |
1 (50%) |
1 (50%) |
2 (100%) |
2 (100%) |
1 (50%) |
||||||||||||||||||||||||
| Disease duration: < 6 months (n-30) | DPN | FWA | RFEO | WSL | BB | CH | BH | WR | FP | LV | BV | MBG | DV | PH | WSS | WYG | Sc | ||||||||||||||
|
Ind (n = 1) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
||||||||||||||||||||||||||
|
TT (n = 1) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
||||||||||||||||||||||
|
BT (n = 12) |
9 (75%) |
10 (83.33%) |
9 (75%) |
6 (50%) |
5 (41.7%) |
3 (25%) |
2 (16.7%) |
3 (25%) |
3 (25%) |
1 (8.3%) |
4 (33.3%) |
5 (41.7%) |
5 (41.7%) |
||||||||||||||||||
|
BL (n = 7) |
6 (66.7%) |
6 (66.7%) |
6 (66.7%) |
5 (55.6%) |
4 (44.4%) |
1 (11.1%) |
3 (33.3%) |
1 (11.1%) |
2 (22.2%) |
2 (22.2%) |
1 (11.1%) |
3 (33.3%) |
4 (44.4%) |
2 (22.2%) |
3 (33.3%) |
||||||||||||||||
|
LL (n = 8) |
4 (50%) |
3 (37.5%) |
2 (25%) |
1 (12.5%) |
4 (50%) |
1 (12.5%) |
2 (25%) |
1 (12.5%) |
1 (12.5%) |
3 (37.5%) |
3 (37.5%) |
3 (37.5%) |
1 (12.5%) |
||||||||||||||||||
|
HL (n = 1) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
||||||||||||||||||||||
|
T1R (n = 3) |
3 (100%) |
1 (33.33%) |
3 (100%) |
1 (33.3%) |
2 (66.7%) |
1 (33.3%) |
3 (100%) |
3 (100%) |
1 (33.3%) |
3 (100%) |
2 (66.7%) |
3 (100%) |
|||||||||||||||||||
|
T2R (n = 3) |
3 (100%) |
1 (33.3%) |
3 (100%) |
1 (33.3%) |
3 (100%) |
1 (33.3%) |
1 (33.3%) |
3 (100%) |
1 (33.3%) |
2 (66.7%) |
1 (33.3%) |
2 (66.7%) |
3 (100%) |
1 (33.3%) |
|||||||||||||||||
|
Site of involvement: Facial (n-12) |
|||||||||||||||||||||||||||||||
| DPN | FWA | RFEO | WSL | BB | CH | BH | WR | FP | LV | BV | MBG | DV | PH | WSS | WYG | Sc | |||||||||||||||
|
BT (n = 8) |
4 (50%) |
4 (50%) |
3 (37.5%) |
1** (12.5%) |
1 (12.5%) |
1 (12.5%) |
2 (25%) |
4 (50%) |
3 (37.5%) |
3 (37.5%) |
1 (12.5%) |
4 (50%) |
3 (37.5%) |
2 (25%) |
|||||||||||||||||
|
LL (n = 4) |
1 (25%) |
1 (25%) |
|||||||||||||||||||||||||||||
|
T2R (n = 1) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
||||||||||||||||||||
|
Site of involvement: Extra-facial (n-41) |
|||||||||||||||||||||||||||||||
| DPN | FWA | RFEO | WSL | BB | CH | BH | WR | FP | LV | BV | MBG | DV | PH | WSS | WYG | Sc | |||||||||||||||
|
Ind (n = 1) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
||||||||||||||||||||||||||
|
TT (n = 1) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
1 (100%) |
|||||||||||||||||||||
|
BT (n = 17) |
16 (94.1%) |
16 (94.11%) |
17 (100%) |
14 (82.4%) |
8 (47.1%) |
8 (47.1%) |
3 (17.6%) |
1 (5.9%) |
4 (23.5%) |
3 (17.6%) |
3 (17.6%) |
4 (23.5%) |
5 (29.4%) |
6 (35.3%) |
|||||||||||||||||
|
BL (n = 9) |
8 (88.9%) |
8 (88.9%) |
7 (77.77%) |
6 (66.7%) |
4 (44.4%) |
1 (11.1%) |
4 (44.4%) |
3 (33.3%) |
3 (33.3%) |
2 (22.2%) |
4 (44.4%) |
5 (55.6%) |
3 (33.3%) |
3 (33.3%) |
|||||||||||||||||
|
LL (n = 10) |
6 (60%) |
4 (40%) |
3 (30%) |
1 (10%) |
5(50%) |
1 (10%) |
5 (50%) |
1 (10%) |
4 (40%) |
7 (70%) |
6 (60%) |
1 (10%) |
|||||||||||||||||||
|
HL (n = 3) |
3 (100%) |
2 (66.7%) |
3 (100%) |
1 (33.3%) |
2 (66.7%) |
2 (66.7%) |
1 (33.3%) |
3 (100%) |
3 (100%) |
1 (33.3%) |
3 (100%) |
2 (66.7%) |
3 (100%) |
||||||||||||||||||
|
T1R (n = 4) |
4 (100%) |
2 (50%) |
4 (100%) |
2 (50%) |
2(50%) |
2 (50%) |
4 (100%) |
3 (75%) |
1 (25%) |
3 (75%) |
1 (25%) |
2 (50%) |
3 (75%) |
||||||||||||||||||
|
T2R (n = 4) |
4 (100%) |
1 (25%) |
3 (75%) |
2(50%) |
2 (50%) |
2 (50%) |
1 (25%) |
3 (75%) |
2 (50%) |
1 (25%) |
3 (75%) |
1 (25%) |
|||||||||||||||||||
p > 0.05 Not significant (BI); p > 0.05 Not significant (Duration); **p < 0.01 (Site);
BB: Brownish background; BH: Broken hairs; BV: Branching vessels; CH: Circle hair; DPN: Distorted pigment network; DV: Dotted vessels; FP: Follicular plugs; FWA: Focal white areas; LV: Linear vessels; MBG: Milky red globules; PH: Pinkish hue; RFEO: Reduced follicular and eccrine openings; Sc: Scales; WYG: Whitish-yellow globules; WR: White rosettes; WSL: Widened skin lines; WSS: White shiny streaks; HL: histoid leprosy; BL: borderline lepromatous; LL: lepromatous; BT: borderline tuberculoid; TT: tuberculoid, T1R: Type 1 reaction; T2R: Type 2 reaction
While in the lepromatous spectrum, in patients with BI = 0/1; focal white areas, a distorted pigment network, reduced follicular and eccrine openings, white shiny streaks, whitish-yellow globules and linear and branching vessels were all seen in equal proportion in single cases; in patients with BI > 1, white shiny streaks were the most common.
Dermatoscopic features based on the site of involvement
Dermatoscopic findings also varied depending on the site of lesions [facial (12) vs. extra-facial (41)] [Table 2]. A statistically significant difference (p < 0.01) was found between the dermatoscopic features of the two groups.
Dermatoscopic features based on the duration of disease
The duration of the disease was broadly divided into less than 6 months and greater than 6 months. In patients with less than 6 months of disease, distorted pigment networks were most common followed by focal white areas. Almost similar findings were noted in long-standing lesions (>6 months, p > 0.05) [Table 2].
Discussion
Leprosy is a chronic granulomatous disorder capable of disguising itself in multiple forms and often poses a diagnostic dilemma to dermatologists. Dermatoscopy is a non-invasive and quick yet accurate method for making a correct diagnosis in many infectious and inflammatory dermatoses, including granulomatous conditions.
Pigmentary changes
Among the various dermatoscopic patterns in previously conducted studies,1,2 whitish-yellow globules is reported to be the most common finding. However, in our patient cohort distorted pigment network was seen in 48 (90.6%) lesions, making it the most common finding, followed by reduced follicular and eccrine openings in 43 (81.1%) lesions. This disparity could be due to previous studies being from a single centre, wherein the dermatoscopic features would be influenced by the prevailing skin phototype of the study population. The present study was a multicentric study covering a more representative pan-India population. Furthermore, the pigment network under dermatoscopy is bound to be prominent in skin of colour, which impairs visualisation of structures below the epidermis.3 A large proportion of patients in prior studies belonged to the BT spectrum.4 In our patient cohort, 23/53 (43.4%) patients were in the lepromatous group, thus covering the lacunae in literature on dermatoscopic features in multibacillary leprosy.
Focal white areas were observed predominantly in the BT spectrum irrespective of BI, site and duration of disease. Thus, it is a definitive dermatoscopic feature of leprosy. This observation reinforces the findings from previous reports.1,4,5 Another feature ‘widened skin lines’ was found in the majority of BT/ BT downgrading to BL cases. This was a unique feature of the present study cohort. Dry patches/plaques and atrophic epidermis with reduced melanin probably produce widened skin lines. The stage of the disease and level of granuloma also influence the dermatoscopic findings.6
Brownish background because of the melanin curtain in the epidermis was another striking feature found in the BT spectrum, predominantly in extra-facial, flat lesions. This is in accordance with reported studies of leprosy in skin of colour.2,7,8 Dermatoscopically, clofazimine-induced pigmentation shows a bluish-black background, pigment dots, a honeycomb pattern and yellow-to-white globules.2 Two patients in our cohort showed the presence of clofazimine-induced bluish pigmentation while on therapy [Figure 2d].
Follicular changes
Follicular and eccrine openings appear as white dots under dermatoscopy,9 which are retained in other granulomatous diseases.10 In our study, reduced appendages were seen in higher proportions, in extra-facial and lesions with >1 BI as compared to facial and lesions with 0/1 BI. This disparity is probably due to pronounced tissue damage by granulomas in cases with >1 BI, and a greater number of follicular and eccrine openings on the face.
Circle hair represents regrowing hair.11 Circle hairs were found predominantly in the tuberculoid spectrum (12 in TT/BT and 3 in BL/LL spectrum), making it a rather specific finding [Figure 2b]. None of the previous reports have described this entity. Circle hair could be a manifestation of an altered follicular milieu in the tuberculoid spectrum or due to the resolution of perifollicular granulomas in patients on treatment.
Broken hairs represent hairs that are cut midway, and they appear as short terminal hairs or black dots.11 In the present study, they were found in 17 lesions (9 in the TT/BT and 6 in the BL/LL spectrum) [Figure 1c] including two cases of histoid leprosy (HL).
White rosettes are due to optical phenomenon of polarised light with narrowing and hyperkeratosis of the infundibulum.12 Recently, these were described in lepromatous leprosy and BL leprosy with T1R.13,14 In this study, two cases of HL demonstrated white rosettes, which were not described in HL previously [Figure 3b].
Follicular plugs were observed in HL [Figure 3a], BL and BT with T1R cases [Figure 2a]. These findings are in line with the previous report.2 This could be due to partial destruction of the hair follicle with hyperkeratosis in the HL/BL and T1R spectrums.
Vascular changes
Vascular structures like linear, branching and crown vessels in granulomatous dermatoses could be attributed to the pushing up of the vessels to the surface by the underlying granulomatous infiltrate.1,6,15,16 Vascular structures found in our study included linear vessels (26 lesions), branching vessels (16 lesions) and dotted vessels (2 lesions). Vessels in T1R are described as short linear blurred vessels in a case series, due to pronounced inflammation and loss of granuloma organisation.17 We could also find blurry vessels in the present study [Figure 2a].
Crown vessels were described specifically in the HL spectrum in previous studies.18,19 In this study, they were not observed, which may be due to the small number of HL cases in the study cohort. Milky red globules are vascular globules that are whitish-red in colour.20 In leprosy, they are described only in reactional leprosy.5 In the present study, they were seen in both reactional and non-reactional cases, especially in lesions with >1 BI and in extra-facial sites.
In similar lines, the pinkish hue also denotes the florid inflammation with widespread vasodilation. However, it was found in the cases with lesser BI, lesions on the extra-facial sites, and lesions with lesser duration of disease.
Morphological structures
Yellow-orange globules/structures do not typify any particular granulomatous condition. In this study, they were found in almost all spectrums, which is in line with other studies.1,2,7 Interestingly, their frequency did not vary according to BI and duration of disease. However, they were more predominant in extra-facial lesions.
White shiny streaks are due to collagen (fibrosis) and are well appreciated in polarised light. In leprosy, fibrosis is profound in the LL/HL spectrum, hence, white shiny streaks were predominantly found in LL/HL. This is in coherence with previous reports.1
Scales
Scales give a clue to the diagnosis of many inflammatory dermatoses.3 In this study, scales were more predominant in the BT spectrum as compared to the LL spectrum.
Lepra reactions
Due to intense inflammation, prominent vascular structures are expected, although edema and cellular infiltrate hinder the visualisation of vessels.21 Other features include a pinkish hue, broken hairs, follicular plugs and scales. Similar findings were noted in the present study.
T2R dermatoscopy demonstrated milky-red areas with pinkish hue. Individual vessels, such as linear vessels or branching vessels or dotted vessels, were not observed. Nevertheless, one case revealed linear vessels. A loosely organised (not compact) granuloma is the reason for this finding.
Limitations of the study
Small sample size and lack of dermatoscopic-histologic correlation were the limitations of this study.
Conclusion
Dermatoscopy is a practical, non-invasive device to assess skin lesions of leprosy and provide cues towards spectral classification. Patches and plaques of the tuberculoid spectrum demonstrate loss of hair follicles and eccrine ducts, circle hairs and vessel prominence, follicular plugging and scales. Pigmentary disturbances, whitish to yellowish globules, widened skin lines when seen in various combinations, are specific dermatoscopic features of leprosy and help to differentiate it from other granulomatous diseases.
Ethical approval
Ethical approval was obtained from the Institutional Ethics Committee, SNMC, Bagalkot, Karnataka; SNMC/IECHSR/2023/A-118/1.0.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Dermatoscopy in leprosy and its correlation with clinical spectrum and histopathology: A prospective observational study. J Eur Acad Dermatol Venereol. 2019;33:1947-51.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy in Leprosy: A clinical and histopathological correlation study. Dermatol Pract Concept. 2021;11:e2021032.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- International dermoscopy society criteria for non-neoplastic dermatoses (general dermatology): Validation for skin of color through a Delphi expert consensus. Int J Dermatol. 2022;61:461-71.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy of borderline tuberculoid leprosy. Int J Dermatol. 2018;57:74-6.
- [CrossRef] [PubMed] [Google Scholar]
- Infective diseases. In: Ankad BS, Mukherjee SS, Nikam BP, eds. Dermoscopy-histopathology correlation by. Singapore: Springer Nature publications; 2021. p. :279-334.
- [Google Scholar]
- Dermatoscopy of granulomatous disorders. Dermatol Clin. 2018;36:369-75.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatoscopy and clinicopathological correlation in different spectrum of leprosy. Clin Dermatol Rev. 2021;5:65-70.
- [Google Scholar]
- Correlation of dermoscopic and histopathologic patterns in leprosy - A pilot study. Indian Dermatol Online J. 2019;10:663-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Trichoscopy in hair disorders in darker skin: An approach to diagnosis. Clin Dermatol Rev. 2020;4:102-14.
- [Google Scholar]
- Granulomatous disorders. In: Ankad BS, Bhat YJ, Rambhia KD, eds. IADVL Atlas of dermoscopy. New Delhi: Jaypee Brothers Medical Publishers; 2022. p. :68-84.
- [Google Scholar]
- Trichoscopy: A new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651-4.
- [PubMed] [Google Scholar]
- Rosettes and other white shiny structures in polarized dermoscopy: Histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311-3.
- [CrossRef] [PubMed] [Google Scholar]
- White rosettes in borderline lepromatous leprosy: A new observation. J Eur Acad Dermatol Venereol. 2020;34:e329-e31.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy of granuloma annulare: A clinical and histological correlation study. Dermatology. 2017;233:74-9.
- [CrossRef] [PubMed] [Google Scholar]
- Orange color: A dermoscopic clue for the diagnosis of granulomatous skin diseases. J Am Acad Dermatol. 2015;72:S60-3.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy of type 1 lepra reaction in skin of color. Dermatol Pract Concept. 2020;10:e2020083.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Crown vessels and shiny white structures in dermoscopy of histoid leprosy. JAAD Case Rep. 2020;6:1147-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Visual dermatology: Crown vessels in dermoscopy of histoid leprosy. J Cutan Med Surg. 2019;23:333.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular structures in skin tumors: A dermoscopy study. Arch Dermatol. 2004;140:1485-9.
- [CrossRef] [PubMed] [Google Scholar]
- Leprosy type 1 (reversal) reactions and their management. Lepr Rev. 2008;79:372-86.
- [PubMed] [Google Scholar]







