Translate this page into:
A novel compound mutation of SLCO2A1 in a Chinese patient with primary hypertrophic osteoarthropathy
Corresponding author: Dr. Bin Chen, Department of Dermatology, Lujiang People’s Hospital, Lujiang, China. chenbin2005164@126.com
-
Received: ,
Accepted: ,
How to cite this article: Chen B. A novel compound mutation of SLCO2A1 in a Chinese patient with primary hypertrophic osteoarthropathy. Indian J Dermatol Venereol Leprol. 2024;90:511-4. doi: 10.25259/IJDVL_71_2023
Dear Editor,
Pachydermoperiostosis, also known as primary hypertrophic osteoarthropathy (OMIM 167100), is an autosomal recessive disorder, characterised by progressive thickening of bone and skin, resulting in pachydermia that frequently includes thickened scalp, dermal oedema, dermal fibrosis, digital clubbing, coarse facial features and adnexal hyperplasia.1 Primary hypertrophic osteoarthropathy typically presents at puberty and progresses gradually over the next 10–20 years, with a male-to-female ratio of about 7:1. Mutations the 15-hydroxy-prostaglandin dehydrogenase (HPGD, MIM 601688) gene and solute carrier organic anion transporter family member 2A1 (SLCO2A1, 601460), have been implicated in the pathogenesis of primary hypertrophic osteoarthropathy. Dysfunction of SLCO2A1 or HPGD can lead to increased prostaglandin E2 (PGE2) levels, either by decreased degradation due to enzymatic loss or a transporter defect.2
A 31-year-old man, born of non-consanguineous marriage presented with a 14-year history of skin thickening and furrowing, digital clubbing, over-curvature of fingernails, ankle and knee joint swelling, joint pain and functional impairment. Physical examination revealed finger and toe clubbing, large joints swelling, and facial coarseness or greasiness [Figure 1a]. Radiological examination showed obvious cortical hyperostosis in the distal tibia and fibula and periostosis of the diaphysis in the distal left and right radius [Figure 1b]. Forehead skin showed significant sebaceous gland proliferation [Figure 1c]. His parents and brother were normal, but his sister had died at the age of 15 due to aplastic anaemia.
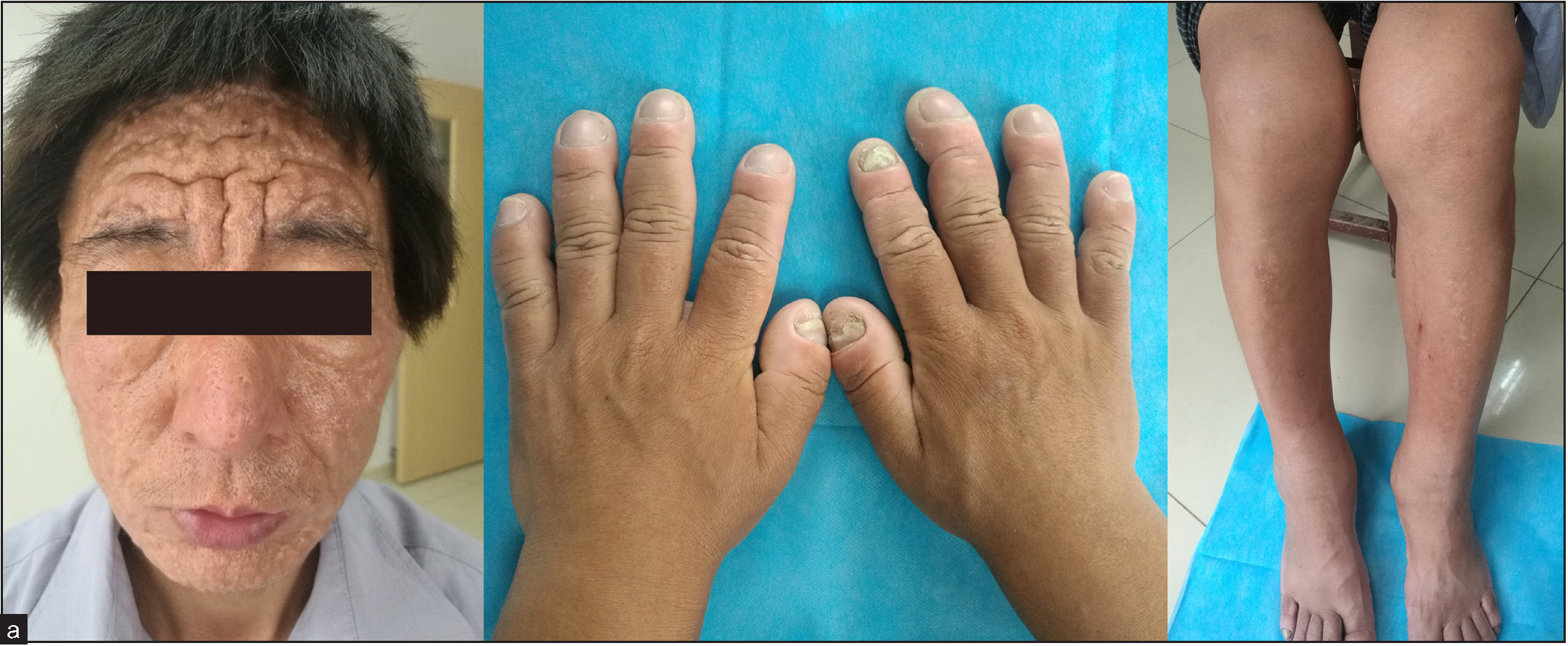
- Forehead skin shows thickening, wrinkles deepening and furrowing (left panel); hands and feet with clubbing and over-curvature fingernails. Bilateral knees and ankles show moderate swelling (middle and right panel).
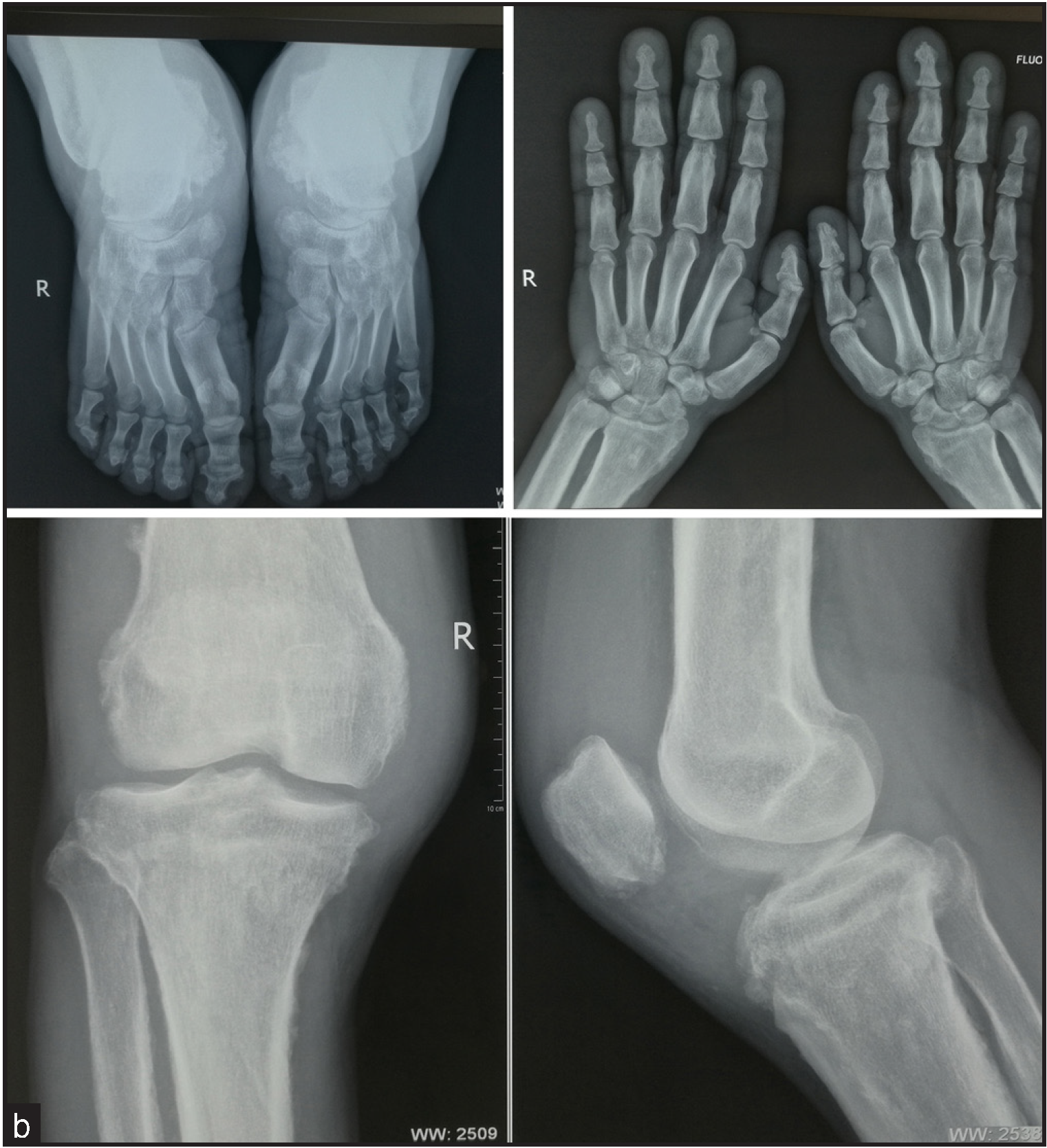
- The feet radiograph shows cortical thickening and acro-osteolysis (top left panel). Hand radiograph shows a loss of the normal tabulation of metacarpals and phalanges and cortical thickening of the metacarpals and the proximal and middle phalanges (top right panel). X-ray of the knee display periosteal hyperostosis, patellae sclerosis, and sclerosis of both the distal femur and tibiofibular (lower panel).
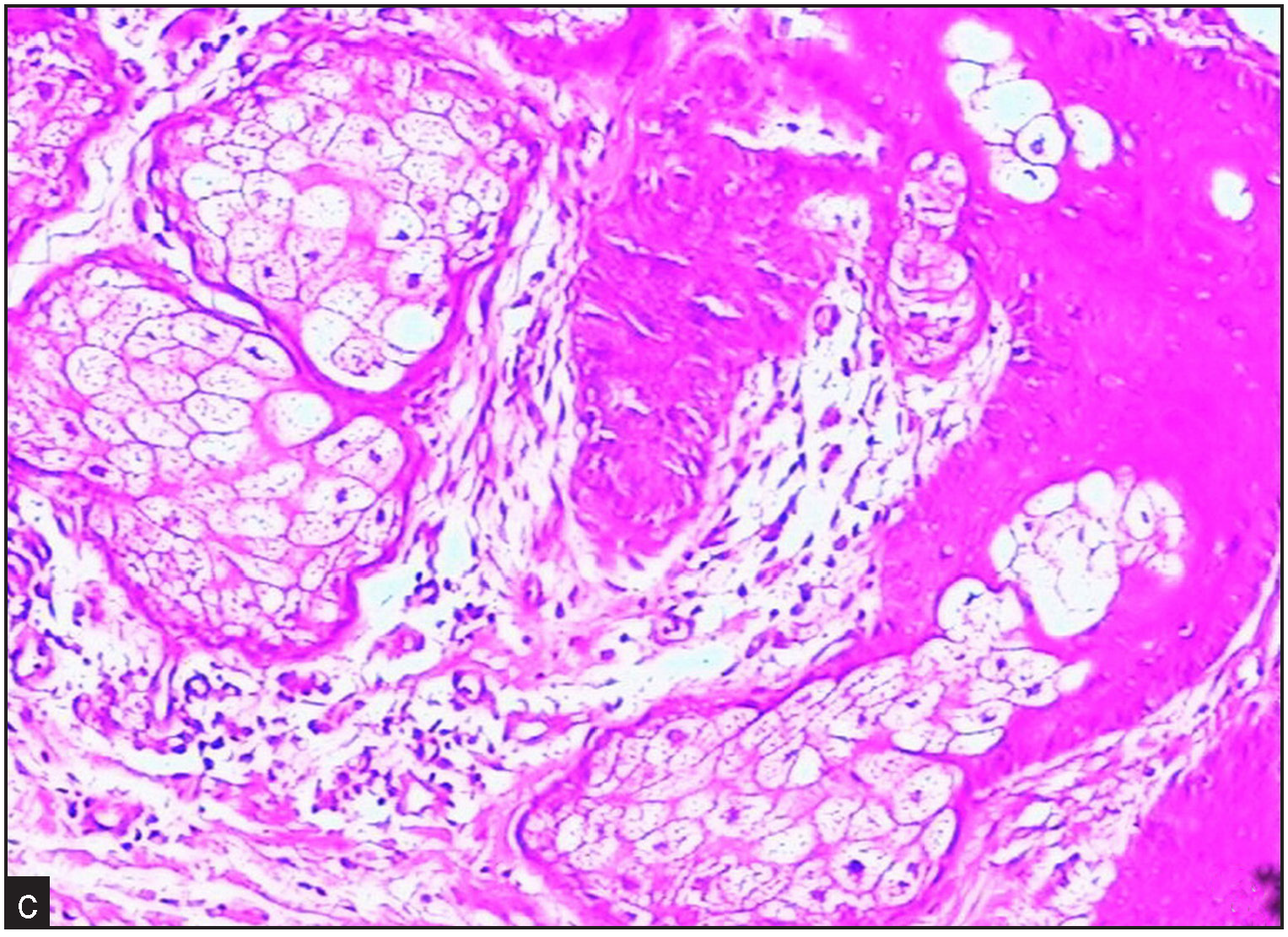
- Forehead skin pathology shows significant sebaceous gland proliferation (Haematoxylin & Eosin, 100x).
We collected whole blood from the proband and his family members to check for mutations in HPGD or SLCO2A1. All members signed the informed consent, and this project was approved by Ethics Committee at the Lujiang County People’s Hospital. The DNA was extracted with Qiagen FlexiGene DNA Kit (No 51206, Hilden, NRW, Germany). The PCR primers were designed to cover all the exons and exon-intron boundaries during the amplification. The primers were designed using primer 3.0 (https://primer3.ut.ee), and the primer sequences are listed in Supplementary Table 1.
The exonic and exon-intron boundary regions of the two genes were amplified and further sequenced with an ABI 3730XL genetic analyser (Thermo Fisher Scientific).
We found a novel compound heterozygous mutation in the SLCO2A1 gene: c.96+4A>C in the exon-intron 2 boundary and c.1807 C>T in exon 13 in the proband [Figure 2]. The c.96+4A>C mutation was located in the splice donor site of intron 2. This mutation might have affected alternative splicing with exon 2, assayed by splicing-based analysis of variants or SPANR (http://tools.genes.toronto.edu/#). The c.1807 C>T mutation introduced a stop codon at position 603 (p.Arg603x) and was predicted to be “PROBABLY DAMAGING” with Polyphen software. The splice site mutation c.96+4A>C was seen in the paternal allele, whereas c.1807 C>T was seen in the maternal allele. The c.96+4A>C mutation was not observed in the HGMD, gnomAD, 1000 Genome Project or ClinVar database, while c.1807 C>T has been previously reported.2
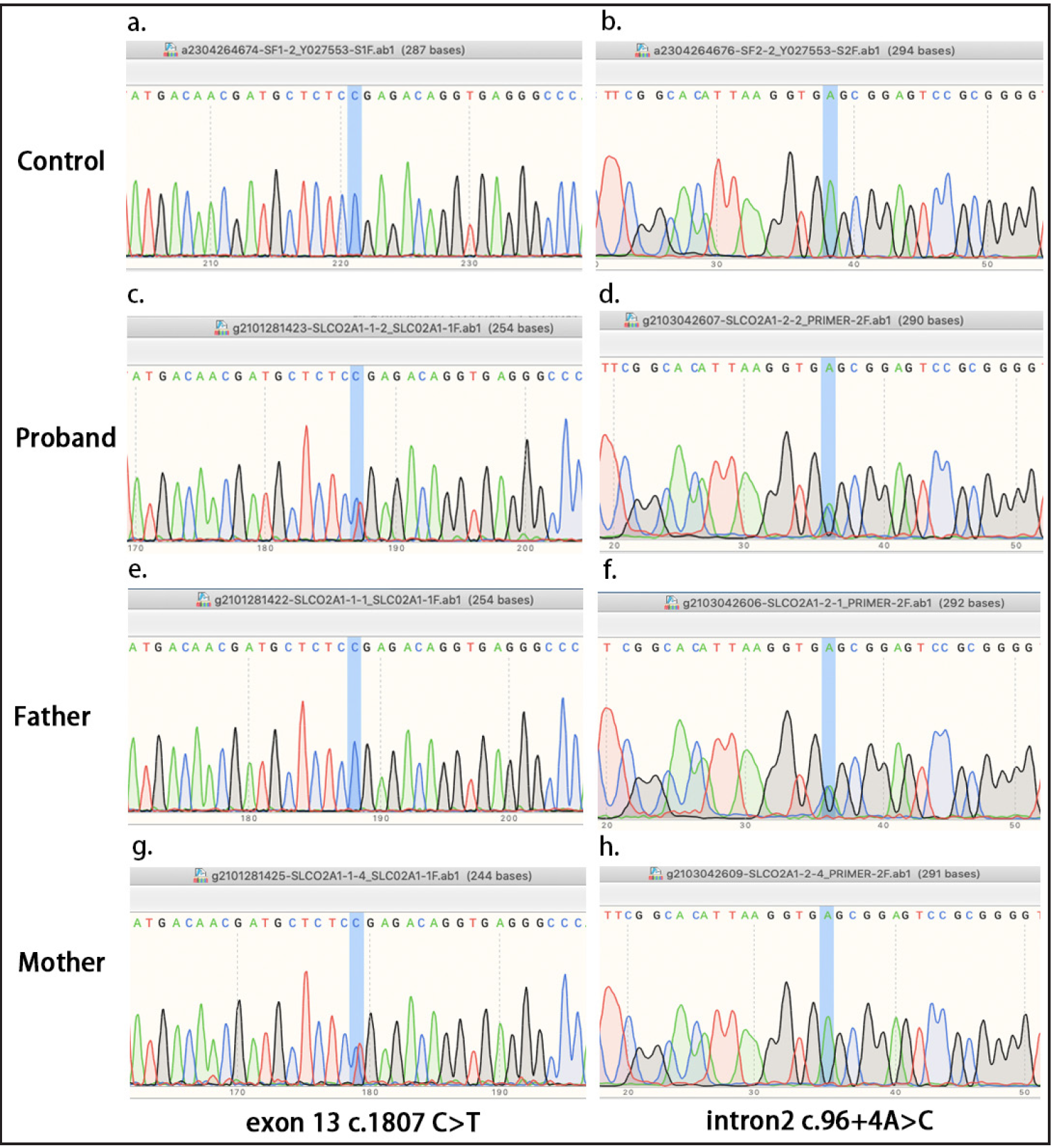
- Mutation analysis of the SLCO2A1 gene. (a–b) No mutations were detected in SLCO2A1 in healthy controls. (c–d) The proband carries the c.96+4A>C in the exon-intron 2 boundary and the c.1807 C>T mutations in exon 13. (e–f) Heterozygous c.96+4A>C intronic splicing mutation in the patient’s father. (g–h) Heterozygous c.1807 C>T exonic mutation in patient’s mother.
The serum samples from the proband, parents and three healthy individuals were collected to determine the concertation of five prostanoids, including PGE2, PGD2, 6keto-PGF1α, 15-keto-PGF2α and PGE1, using targeted metabolomics. The PGE2 level in the primary hypertrophic osteoarthropathy patient (42.1 ng/ml) was more than three times higher than that in his mother (8.29 ng/ml), father (8.73 ng/ml) and healthy controls (9.7 ng/ml). For the other four metabolites, those with greater than 2-fold changes were not found. This finding supported the notion that the dysfunction of PGE2 transportation was dependent on SLCO2A1.
In summary, we identified a novel compound heterozygous mutation in the SLCO2A1 gene, a nonsense mutation p.Arg603X, and a splice-site mutation c.96+4A>C in a Chinese primary hypertrophic osteoarthropathy family. While more than fifty causal mutations have been reported, the correlation between genotypes and phenotypes has yet to be confirmed. One report showed a splicing homozygous c.940+1G>A mutation that potentially correlated with the severity of clinical phenotypes in Japanese patients, which resulted in the entire loss of exon 7 and introduced a premature stop codon.3 More studies are needed to confirm the genotype-phenotype correlation in the Chinese cohort.
The p.Arg603X was previously reported in a Chinese patient, who also carried a p.Gly183Arg missense mutation.4 The proband’s sister did not present a typical primary hypertrophic osteoarthropathy phenotype but died of aplastic anaemia, leading us to suspect she was a carrier of the same compound heterozygous mutation. In agreement with previous findings, female patients with compound heterozygous or homozygous mutations may not have symptoms of primary hypertrophic osteoarthropathy but can present with anaemia and earlier menopause.5 This sex difference might be interpreted by the regulatory function of the prostaglandin transporter encoded by SLCO2A1. The level of prostaglandin regulates the secretion of hormones in females and potentially protects them from being affected. This finding will help us to better understand the aetiology of primary hypertrophic osteoarthropathy.
The limitation of this project is a lack of genotype and phenotype correlation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Mutations in 15-hydroxyprostaglandin dehydrogenase cause primary hypertrophic osteoarthropathy. Nat Genet. 2008;40:789-93.
- [CrossRef] [PubMed] [Google Scholar]
- Three novel mutations in the SLCO2A1 gene in two Chinese families with primary hypertrophic osteoarthropathy. Eur J Dermatol. 2013;23:636-9.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of mutations in the prostaglandin transporter gene SLCO2A1 and its phenotype-genotype correlation in Japanese patients with pachydermoperiostosis. J Dermatol Sci. 2012;68:36-44.
- [CrossRef] [PubMed] [Google Scholar]
- Exome sequencing identifies SLCO2A1 mutations as a cause of primary hypertrophic osteoarthropathy. Am J Hum Genet. 2012;90:125-32.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Differential diagnosis of acromegaly: Pachydermoperiostosis two new cases from Turkey. J Clin Res Pediatr Endocrinol. 2022;14:350-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





