Translate this page into:
Multifocal pyoderma gangrenosum induced by secukinumab in a patient with hidradenitis suppurativa
Corresponding author: Dr. Marcial Álvarez-Salafranca, Department of Dermatology, Miguel Servet University Hospital, Zaragoza, Spain. malvarezs@salud.aragon.es
-
Received: ,
Accepted: ,
How to cite this article: Clemente-Hernández B, Álvarez-Salafranca M, Muelas-Rives I, Ollero-Domenche L, Gracia-Cazaña T, Gilaberte Y. Multifocal pyoderma gangrenosum induced by secukinumab in a patient with hidradenitis suppurativa. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_329_2024
Dear Editor,
A paradoxical reaction during treatment with biologic agents is the unexpected exacerbation or emergence of a medical condition that the biologic therapy is typically intended to treat. The pathogenic mechanisms underlying the paradoxical skin lesions is not yet clear which may involve various immune pathways that get disrupted, leading to an imbalance of cytokines. While most of the paradoxical reactions reported have been associated with the use of TNF-α inhibitors, there is a growing number of cases associated with IL17 inhibitors.1,2
A 67-year-old male with a personal history of nodulocystic acne and Hurley III hidradenitis suppurativa (HS) presented with suboptimal clinical control under intensified treatment with adalimumab 80 mg/week and several intralesional ALA (aminolevulinic acid)-photodynamic therapy sessions. The patient had no history of inflammatory bowel disease. In this context, induction with interleukin-17A inhibitor secukinumab 300 mg at weeks 0, 1, 2, 3, and 4 was initiated. One month after the starting of the treatment, a sudden worsening of the hidradenitis suppurativa lesions was observed with generalised swelling and purulent discharge from the gluteal and axillary lesions. Moreover, despite the introduction of rifampicin and clindamycin in a dose of 300 mg twice daily each, the condition did not improve. Two weeks later, multiple papulopustular, nodular, and cribriform ulcerated lesions with inflammatory edges appeared on his soles, left knee, and left pretibial region respectively [Figures 1a-1b]. Histopathologic examination of the edge of the ulcerated lesion revealed suppurative and necrotising dermatitis with negative staining for microorganisms and therefore, the diagnosis of pyoderma gangrenosum (PG) was made [Figures 2a-2b]. Bone scan showed no alterations and a genetic panel (ExoNIM® clinical study) covering documented genes linked to HS-related autoinflammatory syndromes (PASH, PAPASH, PsAPASH) found no known pathogenic mutation. In this setting, the patient was switched back to the previous adalimumab regimen and the lesions started to improve and were completely healed 6 weeks later [Figure 3].
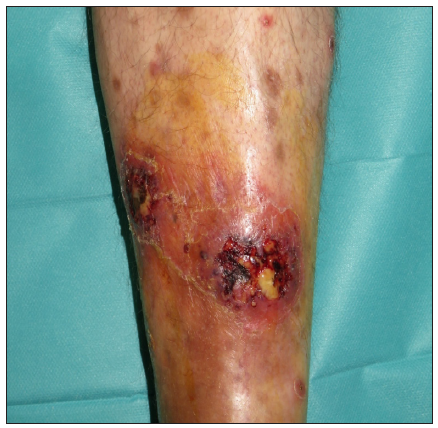
- Multiple papulopustular, nodular, and cribriform ulcerated lesions with violaceous inflammatory edges appeared on his left pretibial region.
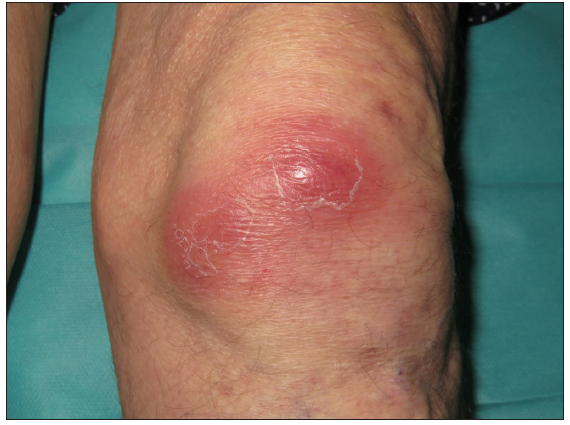
- Erythematous coalescing nodules located on the left knee.
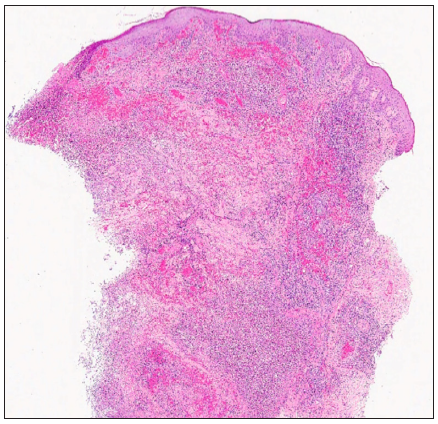
- Suppurative and necrotising dermatitis affecting superficial, middle, and deep dermis with ulceration of the epidermis (Haematoxylin and eosin, 20x).
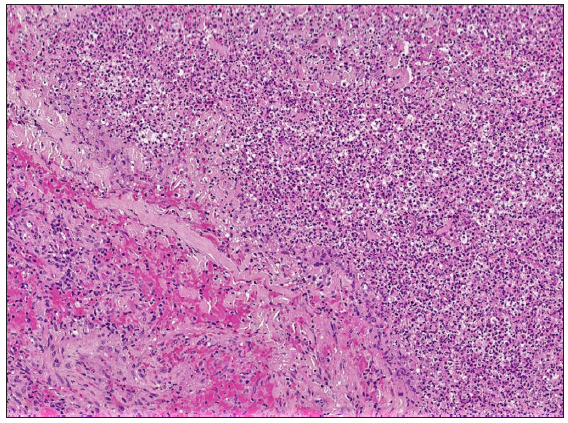
- Diffuse inflammatory infiltrate composed mainly of neutrophils, lymphocytes, and histiocytes with erythrocyte extravasation and central abscess formation (Haematoxylin and eosin, 100x).
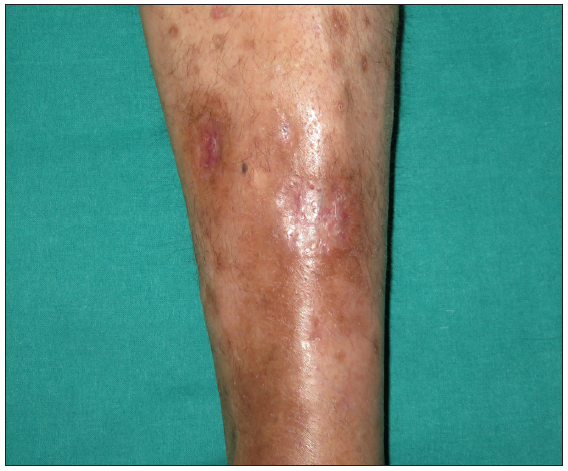
- Pretibial pyoderma gangrenosum healed completely after 6 weeks of secukinumab discontinuation and reintroduction of the intensified adalimumab regimen.
Paradoxical reactions during biologic therapy were initially described as isolated cases in patients with rheumatologic diseases, psoriasis, and inflammatory bowel disease with TNF-alpha inhibitors. Subsequently, paradoxical reactions to other groups of drugs have been described, including IL17 inhibitors. The aetiopathogenic mechanism is complex, based on the involvement of multiple immunological pathways triggering an imbalance of cytokines.1,3 Different immune-mediated adverse effects related to the inhibition of the IL17A/IL17R pathway have been described. These reactions have been classified as hypersensitivity reactions and those secondary to a cytokine imbalance (type b and c adverse reactions to biologic agents). The most frequent adverse effects are paradoxical psoriasis and atopic eczema, although other skin manifestations such as neutrophilic dermatoses, hypersensitivity reactions, and vasculitis, among others, have also been described.1 Moreover, several cases of paradoxically induced HS have been reported, mainly in patients with psoriasis.3 This is particularly relevant in view of the recent European Medicines Agency (EMA) approval of this drug for the treatment of HS.
IL17 is a key cytokine for neutrophil recruitment and activation. Therefore, IL-17 may mediate protective innate immunity against pathogens or contribute to the pathogenesis of several inflammatory diseases, including neutrophilic dermatoses. In this sense, IL-17 inhibitors have been shown to be effective in the treatment of PG.1 However, IL17 inhibitors have been linked to the paradoxical development of neutrophilic dermatoses. It has been hypothesised that IL1-7 inhibition may lead to a compensatory elevation of IL23 levels which could subsequently trigger neutrophil activation and, therefore, may contribute to the development of PG lesions as a paradoxical reaction.1 Furthermore, inhibition of IL-17A by secukinumab can lead to compensatory increase in the levels of IL-17 subtypes such as IL-17C1,2
Although rarely reported, most cases of paradoxical pyoderma gangrenosum secondary to secukinumab have been documented in patients with psoriasis4-8 [Table 1]. Both TNF-alpha and IL17 inhibitors have been shown to be effective therapeutic approaches for the management of PG. However, the management of paradoxical PG in patients under biologic therapy can pose a therapeutic challenge. In this setting, a good response has been described with colchicine, prednisolone, cyclosporin A, risankizumab, or ustekinumab.4-8
| Reference | Underlying disease | Previous treatment | Age/Gender | Latency | Treatment |
|---|---|---|---|---|---|
| Orita et al., 20227 | Psoriasis | Adalimumab | 68, M | 3 years | Risankizumab |
| Wollina et al., 20206 | Psoriasis | NA | 33, F | 14 months | Prednisolone |
| Petty et al., 20205 | Psoriasis | Adalimumab | 50, F | 3 weeks | Cyclosporine, prednisone, infliximab (stopped due to secondary leukocytoclastic vasculitis), ustekinumab |
| Jin et al., 20194 | Psoriasis | Adalimumab | 47, F | 4 months | Cyclosporin |
| Avci et al., 20238 | Hidradenitis suppurativa | Adalimumab | 43, M | 10 days after 6th dose | Piperacillin-tazobactam and colchicine |
| Clemente-Hernández et al. (present case) | Hidradenitis suppurativa | Adalimumab | 67, M | 6 weeks | Adalimumab |
NA: not available. M: male. F: female.
To our knowledge, we report the second case of paradoxical PG related to secukinumab in a patient with HS. It can be hypothesised that an increase in IL23 levels due to IL17A inhibition can promote neutrophil recruitment and activation, leading to this clinical scenario. As IL17 inhibitors become part of the therapeutic armamentarium for HS patients, dermatologists should be aware of the occurrence of paradoxical events in these patients, including PG.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- The dark side of the moon: The immune-mediated adverse events of IL-17A/IL-17R inhibition. J Dermatolog Treat. 2022;33:2443-54.
- [CrossRef] [PubMed] [Google Scholar]
- Paradoxical reactions to biologic therapy in psoriasis: A review of the literature. Actas Dermosifiliog. 2018;109:791-800.
- [CrossRef] [PubMed] [Google Scholar]
- Secukinumab-induced paradoxical hidradenitis suppurativa. Dermatol Ther. 2020;33:e13150.
- [CrossRef] [PubMed] [Google Scholar]
- Pyoderma gangrenosum triggered by switching from adalimumab to secukinumab. J Dermatol. 2019;46:e108-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pyoderma gangrenosum induced by secukinumab in a patient with psoriasis successfully treated with ustekinumab. JAAD Case Rep. 2020;6:731.
- [CrossRef] [PubMed] [Google Scholar]
- Pyoderma gangrenosum induced by secukinumab-A late paradoxical drug reaction. Dermatol Ther. 2020;33:e13161.
- [CrossRef] [PubMed] [Google Scholar]
- Pyoderma gangrenosum caused by secukinumab successfully treated with risankizumab: A case report and literature review. Clin Exp Dermatol. 2022;47:1372-4.
- [CrossRef] [PubMed] [Google Scholar]
- Pyoderma gangrenosum and Behçet’s-like disease induced by secukinumab: A paradoxical drug reaction. J Dermatolog Treat. 2023;34:2235040.
- [CrossRef] [PubMed] [Google Scholar]






