Translate this page into:
Systemic tofacitinib in paediatric alopecia areata
Corresponding author: Dr. Rachita S Dhurat, Department of Dermatology, Lokmanya Tilak Municipal General Hospital and Medical College, Sion, Mumbai, Maharashtra, India. rachitadhurat@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Dhurat RS, Sharma R, Zatakia S, Ranka A. Systemic tofacitinib in paediatric alopecia areata. Indian J Dermatol Venereol Leprol. 2024;90:811-3. doi: 10.25259/IJDVL_1109_2023
Dear Editor,
There is paucity of data on the use and safety of tofacitinib in the paediatric population for alopecia areata, especially from the Indian subcontinent. We report our experience in 20 children with alopecia areata treated with tofacitinib at a tertiary care hospital in India. This was a retrospective study in which hospital records were reviewed to identify patients with alopecia areata less than 18 years of age treated with oral tofacitinib between January 2021 and March 2023. The severity of alopecia areata was assessed using Severity of Alopecia Tool (SALT) in which a SALT score of 100 corresponds to the complete absence of scalp hair and a SALT score of 0 indicates complete hair growth.1 Response to treatment was assessed after 6 months of therapy by calculating the percent change in SALT score from baseline to the most recent follow-up. Study participants were divided into two groups on the basis of treatment response: non-responders who achieved a percentage change in SALT score of <5% and responders who achieved a percentage change in SALT score of ≥5%.2,3 Responders were further divided into four groups according to their percentage improvement in SALT score: 5%–25% (modest response), 26%–50% (moderate response), 51%–75% (significant response) and 76%–100% (markedly significant response).
Twenty children with alopecia areata were treated with tofacitinib (9 boys and 11 girls) for a median duration of 7 months (mean 9.45 months; range 6–26 months). The age at the time of initiation of treatment ranged from 3 years to 17 years (mean 10.8 years). Among the morphological types of alopecia areata, seven (35%) had a reticular type, five (25%) had alopecia subtotalis, five (25%) had patchy alopecia and three (15%) had alopecia totalis. The baseline SALT score at the time of starting tofacitinib was > 50 in thirteen patients and <50 in seven patients. Of these seven patients with baseline SALT score < 50, two had SALT score < 20. These two patients were started on tofacitinib after there was no response to topical therapy, intralesional steroids, and immunotherapy for more than 6–8 months. The disease duration varied from 2 months to 7 years with a median duration of 18 months and a mean duration of 28 months. None of the patients had any associated co-morbidities but two patients had a family history of alopecia areata. Study participants characteristics have been summarised in Table 1. Pre-treatment investigations were performed in all patients. No active or latent infections or other significant metabolic disturbances were found.
| Patient who completed a minimum 6 months treatment, n = 20 | ||||
|---|---|---|---|---|
| All patients, n = 20 | Responders, n = 20 | Patient with baseline SALT score > 50, n = 13 | Patient with baseline SALT score < 50, n = 7 | |
| Age, y, median (range) | 11 (3–17) | 11 (3–17) | 9 (4–17) | 14 (3–17) |
| Sex, n (%) | ||||
| Male | 9 (45) | 9 (45) | 7 (53.84) | 2 (29) |
| Female | 11 (55) | 11 (55) | 6 (46.16) | 5 (71) |
| Age of alopecia areata onset, y, median (range) | 8.25 (1–17) | 8.25 (1–17) | 6 (1–16) | 10 (3–17) |
| Duration of disease, median (range) | 18 (2–84) | 18 (2–84) | 12 (2–72) | 24 (3–84) |
| Subtype, n (%) | ||||
| Patchy alopecia areata | 5 (25) | 5 (25) | 0 | 5 (71.4) |
| Reticular alopecia areata | 7 (35) | 7 (35) | 5 (38.4) | 2 (28.6) |
| Alopecia subtotalis | 5 (25) | 5 (25) | 5 (38.4) | 0 |
| Alopecia totalis | 3 (15) | 3 (15) | 3 (23.1) | 0 |
| Eyebrow/eyelash involvement | 7/20 (35) | 7/20 (35) | 7/13(53.8) | 0 |
| Nail diseases, n (%) | 0 | 0 | 0 | 0 |
| Autoimmune comorbidities, n (%) | 0 | 0 | 0 | 0 |
| Atopic disease, n (%) | 0 | 0 | 0 | 0 |
| Family history of alopecia, n (%) | 2 (10) | 2 (10) | 2 (15.3) | 0 |
The dose of tofacitinib was decided according to the weight of the patient.4 The dose varied from 2.5 mg to 10 mg/day, with a mean dose of 5.75 mg/day. Eight patients received 2.5 mg/day, five patients received 5 mg/day and seven patients received 10 mg/day.
All twenty patients showed a percentage change in SALT score of ≥5 % and thus all were responders. The percentage change in SALT score varied from 5.17 to 100% with a median of 89.4 % and a mean of 75.8 %. Among all patients, 15 (75%) showed markedly significant improvement, two (10%) showed significant improvement, two (10%) responded moderately and one (5%) showed modest improvement. Overall, 85% (17/20) achieved over 50% improvement in SALT score from baseline and 35% (7/20) achieved complete regrowth (SALT score of 0). A visible reduction in SALT score was seen as early as 1.5 months in one patient. Most of the patients (18/20) showed visible regrowth in 2–5 months of therapy; a single patient showed a visible response at 8 months of therapy.
Response characteristics of the patients are presented in Table 2. Representative photographs of patients who responded to therapy are presented in Figures 1 and 2.
| All patients, n = 20 | Responders, n = 20 | Patient with baseline SALT > 50, n = 13 | Patient with baseline SALT < 50, n = 7 | |
|---|---|---|---|---|
| Initial SALT score – median; mean (range) | 56.7; 57.53 (8.7–100) | 56.7; 57.53 (8.7–100) | 67.6; 73.57 (52.8–100) | 29.6; 27.76 (8.7–46) |
| Most recent SALT score – median; mean (range) |
4.8; 11.64 (0–70.16) |
4.8; 11.64 (0–70.16) |
8.97; 17.20 (0–70.16) |
0; 2.88 (0–11) |
| % change in SALT score (%) – median, mean (range) | 89.4; 75.84; (5.17–100) | 89.4; 75.84; (5.17–100) |
89.4; 77.79 (5.17–100) |
100;86.05 (44.44–100) |
| % change in SALT score <5%, n (%) | 0 | 0 | 0 | 0 |
| % change in SALT score 5%–25%, n (%) | 1 (5) | 1 (5) | 1 (7.7) | 0 |
| % change in SALT score 26%–50% n (%) | 2 (10) | 2 (10) | 1 (7.7) | 1 (14.3) |
| % change in SALT score 51%–75% | 2 (10) | 2 (10) | 1 (7.7) | 1 (14.3) |
| % change in SALT score 76%–100%, n (%) | 15(75) | 15(75) | 10 (77) | 5 (71.4) |
| Treatment duration, months – median, mean (range) | 7; 9.45 (6–26) | 7; 9.45 (6–26) |
8;10.6 (6–26) |
7; 8.2 (6–15) |
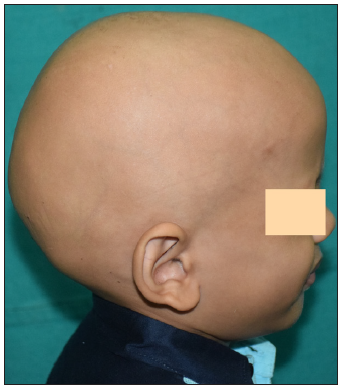
- Baseline image of a 4-year-old boy with alopecia totalis before starting tofactinib.
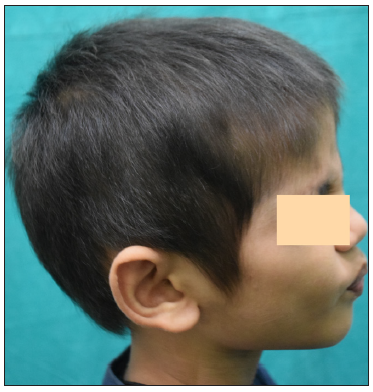
- Post 4 months of treatment with tofacitinib.
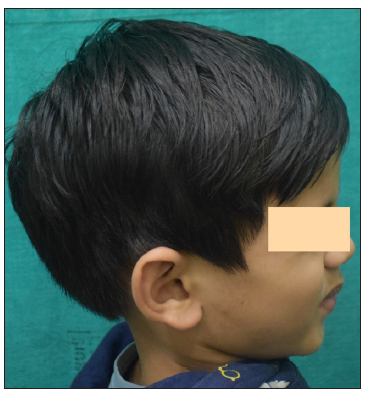
- Post 6 months of treatment with tofacitinib.
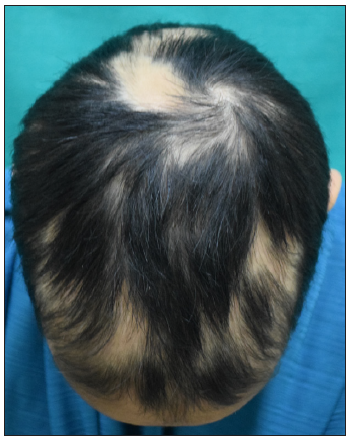
- Baseline image of a 12-year-old girl with alopecia areata.
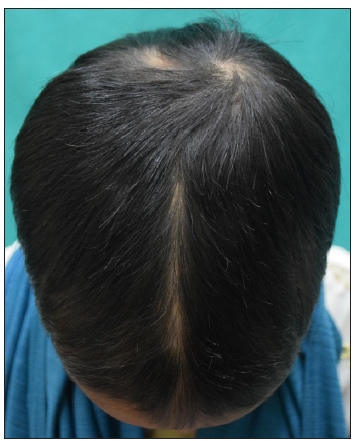
- Post 4 months of treatment with tofacitinib.
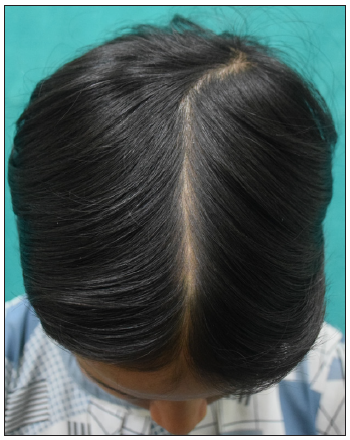
- Post 9 months of treatment with tofacitinib.
Adverse events were mild and included headaches (two patients) and dizziness (one patient), which subsided with continuing therapy. One patient had a transient fall in haemoglobin from 13.3 g/dl to 10.0 g/dl, which improved with oral haematinics.
We also observed that out of total seven patients who achieved complete regrowth (SALT score 0), the baseline SALT score was <50 in four patients and > 50 in three patients. In these four patients (with baseline SALT<50), tofacitinib was tapered and even stopped without much flare of disease activity. The relapses seen if any after stopping tofacitinib were mild and localised and were managed with topical/intralesional steroids. None of these patients required restarting of tofacitinib. On the other hand, in the other three patients who achieved complete regrowth and had baseline SALT > 50, whenever tapering of tofacitinib was attempted, there was a flare of disease activity with multifocal hair pull test positivity.
To conclude, tofacitinib is a promising therapy for alopecia areata in children and adolescents. Since alopecia areata can show spontaneous regrowth, the efficacy of tofacitinib can only be definitively determined by performing randomised controlled trials.
Ethical approval
The Institutional Review Board approval is not required as this was a retrospective study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Alopecia areata in-vestigational assessment guidelines. J Am Acad Dermatol. 1999;40:242-6.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32.
- [CrossRef] [PubMed] [Google Scholar]
- Juvenile idiopathic arthritis treatment updates. Rheum Dis Clin North Am. 2021;47:545-63.
- [CrossRef] [PubMed] [Google Scholar]





