Translate this page into:
Paradoxical eczema in psoriasis patients following treatment with secukinumab
Corresponding author: Dr. Shekhar Neema, Department of Dermatology, Base Hospital, Lucknow, Uttar Pradesh, India. shekharadvait@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Neema S, Pathania V, Nyalam P, Kamboj P, GB P. Paradoxical eczema in psoriasis patients following treatment with secukinumab. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_898_2024
Dear Editor,
Psoriasis is a chronic immune-mediated inflammatory disease of unknown aetiology, with various biological therapies approved for its management. These biologics include TNF α inhibitors (etanercept, adalimumab, infliximab and certolizumab), IL-17 inhibitors (secukinumab, ixekizumab and brodalumab), IL-12/23 inhibitors (ustekinumab) and IL-23 inhibitors (guselkumab, tildrakizumab and risankizumab). Secukinumab, an IL-17 inhibitor, was the first in class, approved in 2015 for treating moderate to severe chronic plaque psoriasis.1 Although the side effects are uncommon, the most frequently reported include nasopharyngitis and headache.2,3 Cutaneous adverse events (CAE) reported during biologic therapies include skin infections, paradoxical psoriasis, pustular dermatoses, and eczema.4 In this respect, we describe three cases of paradoxical eczema in psoriasis patients after secukinumab administration. The case details are tabulated in Table 1.
| Sr No | Age/Duration (years) | Treatment history | Atopy | Eczema | Treatment | |
|---|---|---|---|---|---|---|
| Interval after starting secukinumab | Site | |||||
| Case 1 | 63/7 |
MTX CsA Acitretin |
Nil | 2 weeks | Trunk |
Prednisolone 30 mg OD CsA 5mg/Kg Secukinumab stopped |
| Case 2 | 49/12 |
MTX Etanercept |
Nil | 3 weeks | Scrotum | Topical steroid and emollient |
| Case 3 | 65/25 |
MTX CsA Acitretin Etanercept Infliximab |
Nil | 12 weeks | Legs |
CsA 5 mg/Kg Secukinumab stopped |
MTX - methotrexate; OD - daily; CsA – cyclosporine
The first case includes a 63-year-old man with a seven-year history of chronic plaque psoriasis, presenting with a recent flare affecting 60% of the body surface area (BSA). He was treated intermittently with methotrexate, cyclosporine, and acitretin in the last seven years. Treatment with injection secukinumab was initiated at a dose of 300 mg subcutaneously weekly. However, after two weeks, there was an aggravation of symptoms with oozing and involvement of 80% BSA. A histopathological examination revealed spongiotic dermatitis [Figure 1]. Secukinumab was discontinued, and the patient was managed with a tapering course of oral steroids (prednisolone started at 30 mg once a day and tapered slowly over 4 weeks) and cyclosporine (5 mg/kg). Significant improvement was observed at four weeks.
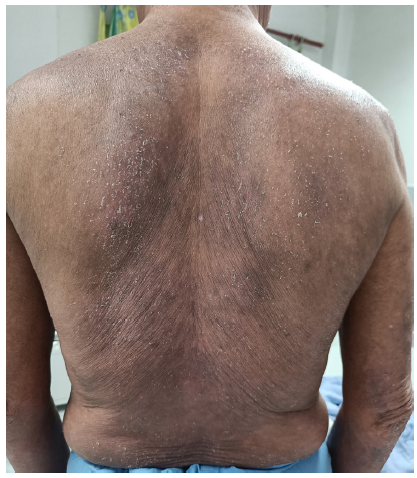
- Diffuse erythematous scaly plaques with excoriations on the trunk.
The second case involved a 49-year-old man with a 12-year history of chronic plaque psoriasis and psoriatic arthritis. He presented with a recent disease flare affecting 70% of the body surface area. He was previously treated with methotrexate and etanercept. Injection secukinumab (300 mg, subcutaneously, weekly) was started, leading to significant improvement in skin and joint symptoms after three weeks. However, the patient developed scrotal dermatitis [Figure 2]. He was treated with emollients twice a day and topical fluticasone 0.05% cream once a day. His symptoms resolved and Secukinumab was continued as per standard dosing protocol.
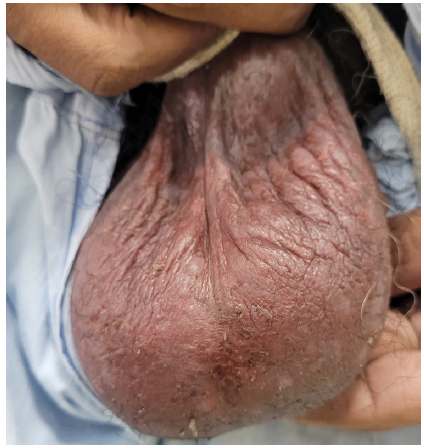
- Erythematous oozy plaques on the scrotum.
The third case includes a 65-year-old man with a 25-year history of chronic plaque psoriasis with psoriatic arthritis. He presented with active disease and a recent flare affecting 25% of the body surface area. He was treated previously with methotrexate, cyclosporine, etanercept, infliximab, and acitretin. Secukinumab 300 mg subcutaneously, administered weekly, was initiated for the flare, resulting in significant improvement in both skin and joint disease. However, after three months, the patient developed eczematous eruptions with oozing on both legs [Figure 3]. Cyclosporine at 5 mg/kg was started but without much improvement at 2 weeks. Secukinumab was discontinued, leading to an improvement in eczema after two weeks.
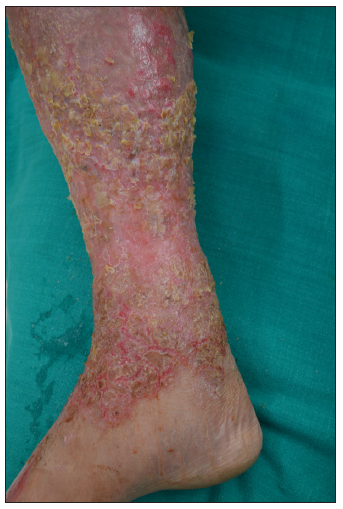
- Erythematous oozy crusted plaques involving the lower half right leg.
The use of biological therapies for psoriasis has been associated with the development of eczema, among other cutaneous adverse events.4 Development of eczema while on anti-TNFα therapy has been well-documented in the literature.5 However, there are only anecdotal cases of eczematous eruptions induced by anti-IL-17 agents in patients with psoriasis.6 The co-occurrence of psoriasis and eczema is rare but has been reported occasionally.7,8
Multiple hypotheses have been described in the literature for developing paradoxical eczema (PE) during biological therapy. The PE may be due to an imbalance in Th1 and Th2 pathways. The inhibition of the Th1/Th17 pathway by secukinumab may exacerbate the Th2 pathway and the consequent appearance of atopic eczema.2,3,6 “Immune or cytokine imbalance syndromes” could also explain the occurrence of eczema on biologics.4 Despite atopic eczema and psoriasis being genetically and immunologically discrete, biological therapy for either condition can lead to a paradoxical phenotypic switch to the other due to cytokine imbalance.8 Eczematous eruption induced by anti-TNF-α agents showed high levels of IL-22. Therefore, IL-22 may be a key mediator in the pathogenesis of eczema induced by anti-IL-17 agents.6
A personal history of atopy could also be a risk factor for paradoxical eczema. Studies have shown that patients with a prior history of atopy may experience eczematous eruptions caused by biologics.4,8 However, no epidemiological studies have confirmed an association between PE and atopy in psoriasis patients.8 If a paradoxical reaction is severe, secukinumab should be discontinued, and an alternative treatment for psoriasis and eczema should be initiated. Mild reactions can be managed with topical steroids, and secukinumab can be continued. It is important to be aware of this rare adverse event with IL-17 inhibitors, which can be misconstrued as a primary or secondary failure. Since IL-17 inhibitors are not used to treat eczema, it is better to term this condition as IL-17 triggered eczema rather than paradoxical eczema.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Secukinumab in the treatment of psoriasis: Patient selection and perspectives. Psoriasis (Auckl). 2018;8:75-82.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Atopic dermatitis as a paradoxical effect of secukinumab for the treatment of psoriasis. Case Rep Dermatol. 2021;13:336-39.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic-like dermatitis after secukinumab injection: A case report. Dermatol Ther. 2019;32:e12751.
- [CrossRef] [PubMed] [Google Scholar]
- Phenotypic switch to eczema in patients receiving biologics for plaque psoriasis: A systematic review. J Eur Acad Dermatol Venereol. 2020;34:1440-48.
- [CrossRef] [PubMed] [Google Scholar]
- Eczema as an adverse effect of anti-TNFα therapy in psoriasis and other Th1-mediated diseases: A review. J Dermatolog Treat. 2017;28:237-41.
- [CrossRef] [PubMed] [Google Scholar]
- Eczematous eruption during anti-interleukin 17 treatment of psoriasis: An emerging condition. Br J Dermatol. 2019;181:604-06.
- [CrossRef] [PubMed] [Google Scholar]
- Concurrence of psoriasis vulgaris and atopic eczema in a single patient exhibiting different expression patterns of psoriatic autoantigens in the lesional skin. JAAD Case Rep. 2018;4:429-33.
- [CrossRef] [PubMed] [Google Scholar]
- Paradoxical eczema in patients with psoriasis receiving biologics: A case series. Clin Exp Dermatol. 2022;47:1174-78.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





