Translate this page into:
Disease severity assessment in hidradenitis suppurativa: A single-centre cross-sectional study utilising clinical evaluation, high-resolution ultrasound and colour doppler
Corresponding author: Dr. Sunil Dogra, Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. sundogra@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Rao S, Sharma A, Kumaran M S, Narang T, Sinha A, Dogra S. Disease severity assessment in hidradenitis suppurativa: A single-centre cross-sectional study utilising clinical evaluation, high-resolution ultrasound and colour doppler. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_542_2024
Abstract
Background
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disorder characterised by recurrent painful nodules, abscesses, and sinus tract formation in intertriginous areas. Accurate staging of this disorder is crucial for appropriate management and prognosis. The role of imaging in the staging of HS is still evolving.
Objective
To assess the correlation between clinical disease severity staging and high-resolution ultrasonography and colour doppler (HRUS-CD) staging in HS patients.
Design and setting
This was a cross-sectional, single-centre study in patients above 18 years of age with the clinical diagnosis of HS. All patients underwent clinical examination and high-resolution ultrasonography and colour doppler study with high-frequency linear probe (L+ 8–24 MHz). Correlations between clinical Hurley staging and the various ultrasonographic parameters i.e. SOS-HS (sonographic staging of severity of HS), degree of vascularisation, fibrotic scarring and oedema were explored and analysed. Spearman’s correlation coefficient was applied to study the correlations and p<0.05 was considered significant.
Results
A total of 46 patients with HS who met the inclusion criteria were included. The majority of patients were clinically in Hurley stages 1 (32.6%; n=15) and 2 (56.5%; n=26) (41 in all). However, on the basis of ultrasonographic findings, significant numbers of patients had greater severity of staging on SOS-HS (58.5%; n=24/41), higher vascularity on colour doppler (26.8%; n=11), more severe grading of fibrous scarring (43.9%; n=18) and oedema (68.2%; n=28) in comparison to the clinical Hurley staging.
Limitation
Limitations of our study include a small sample size and cross-sectional design.
Conclusion
The results of the study indicate that the majority of the patients were under-staged and under-treated clinically, hence emphasising the role of high-resolution ultrasonography and colour doppler study in the accurate determination of HS severity. Future research should focus on standardised protocols and larger, prospective studies to establish the role of these imaging modalities in routine clinical practice.
Keywords
hidradenitis suppurativa
ultrasound
colour doppler
severity
Introduction
Hidradentitis suppurativa (HS) is a chronic inflammatory skin disease of the hair follicle unit with average age of onset during the second and third decades of life. Clinical severity is commonly graded using the Hurley staging system,1,2 that classifies this disorder into three stages of disease severity largely based on the presence of cicatrisation and sinuses. Physical examination potentially underestimates the severity as palpation of lesions is often difficult due to the severe inflammation and associated pain. This often causes misinterpretation of the extent of the lesions, leading to errors in disease severity assessment. Advances in high-resolution ultrasonography (USG) in HS have highlighted the limitations of clinical assessment.3–6
The high-resolution USG can detect the actual type and extent of anatomic abnormalities apart from treatment monitoring, thereby helping to provide detailed structural information which helps with presurgical mapping of lesions. This along with colour doppler (high-resolution USG and colour doppler; HRUS-CD) allows the detection of widened hair follicles, pseudocysts, and fluid collections and tunnels.7 Colour doppler can also help estimate the degree of vascularisation and inflammation in HS.4 In order to delve deeper into the role of high-resolution USG and colour doppler in HS patients, our study was aimed at correlating clinical and ultrasonographic disease severity staging in patients with this disorder.
Methods
This was a cross-sectional, single-centre study in patients of skin of colour (Fitzpatrick skin types IV and V). The study population included patients aged ≥18 years, who met the clinical diagnostic criteria of HS; viz. presence of typical lesions in typical locations and chronicity. Typical lesions included nodules, abscesses, fistulous tracts and scarring, typical locations meant involvement of more than one of the following typical body sites: axillae, genito-femoral area, perineum, gluteal area in both males and females and the inter- or infra-mammary area in females. Chronicity was defined as two recurrences within 6 months.8 Exclusion criteria included patients not giving consent, inability to perform the ultrasound due to severe suppuration and pain and patients with a history of excisional surgery or procedures in the affected area.
Description of clinical and ultrasound examination
The clinical examination was a single-session physical examination for the detection of inflammatory or non-inflammatory nodules, fistulas and abscesses and the sites examined were axillae, infra-mammary, periumbilical, inguinal, buttocks, thighs and perianal regions. Nodules were defined as solid and raised lesions of size ≥ 1 cm. Abscess was defined as a collection of pus in a cavity of size ≥1 cm. Fistulas were defined as abnormal pathologic connections between two sites with normal intervening areas.
The high-resolution USG and colour doppler were performed by an experienced radiologist, and a detailed report was prepared on the abnormalities found in all the affected areas. The USG was done on an ESOATE MYLAB 9 machine with a high-frequency linear probe (L+ 8–24 MHz). The sonographic data was analysed based on the widening of hair follicles, abnormal thickening, echogenicity of the skin layers, types of lesions, lesion location and extent in all axes [Table 1]. To avoid any bias, the assignment of scores was blind in that neither the dermatologist nor the radiologist was aware of each other’s assessment. The comparison between the vascularisation grade and clinical Hurley stage was sought by hypothesising that absent to minimal vascularisation would be seen in stage 1, moderate vascularisation in stage 2 and high vascularisation in stage 3.
| Stage | |
|---|---|
| 1 | Single fluid collection and dermal changes (hypoechoic or anechoic pseudocystic nodules, widening of the hair follicles, alterations in the dermal thickness or echogenicity) affecting a single body segment (e.g. axilla, groin, breast, buttock) (uni- or bilateral) without fistulous tracts |
| 2 | Two to four fluid collections or a single fistulous tract with dermal changes affecting up to two body segments (uni- or bilateral) |
| 3 | Five or more fluid collections or two or more fistulous tracts with dermal changes or involvement of three or more body segments (uni- or bilateral) |
Outcome parameters
The primary outcome measure was the correlation in diagnostic staging between the clinical and USG examinations. The clinical disease staging was determined according to the Hurley staging,2 while the high-resolution USG and colour doppler staging was done using sonographic scoring of HS (SOS-HS)4 by the radiologist separately. Correlation of all the high-resolution USG and colour doppler parameters (SOS-HS staging, degree of vascularisation, type of fistulous tracts, the pattern of vascularity of fistulous tracts, grading of fibrotic scarring and oedema of fistulous tracts)4,9,10 with clinical Hurley staging was assessed.
Data compilation and statistical analysis
Patients were recruited in a consecutive manner from the dermatology clinic. Clinical and high-resolution USG and colour doppler parameters were analysed according to the recommended statistical tests. Comparisons of values of skewed data were done via the Kruskal–Wallis test in cases with >2 groups. For categorical data, comparisons were made using Pearson’s chi-squared test, or Fisher’s exact test, as appropriate. Pearson’s or Spearman’s correlation coefficient was applied to study the correlations between the above-mentioned parameters, and p<0.05 was considered significant.
Results
Forty-six patients met the inclusion criteria and were enrolled in the study. The mean age of patients was 28.07 ± 7.28 years, and 56.5% (n=26) of the patients were men. The majority (67.4%, n=31) of patients were in the age range of 18–30 years. The mean age of onset of disease was 23.48 ± 6.76 years. The mean body mass index (BMI) was 26.82 ± 4.90 kg/m2. The mean number of sites involved was 3.02 ± 1.20. The most commonly affected locations were axilla in 60.9% (n=28) patients and groin in 21.7% (n=10) patients. Most patients (86.9%, n=40) did not have a family history of HS, whereas 13.1% (n=6) reported having an affected first-degree relative. Acne was present in 76.1% (n=35) of the patients during evaluation or was documented in the past. History of premenstrual flare was present in 55% (n=11) of the female patients. A history of smoking was present in 21.7% (n=10) of patients, and alcohol consumption was reported by 28.3% of patients. The mean age of onset of smoking was 21.90 ± 4.93 years, and the mean total duration of smoking was 9.80 ± 4.80 years. The mean age of starting alcohol intake was 22.50 ± 5.13 years, and the mean duration of alcohol intake was 8.25 ± 5.01 years. Twenty-nine patients (63.04%) had tried prior systemic treatment. A complete description of patient demographic characteristics can be found in Table 2.
| Parameters assessed | Mean ± SD || Frequency (%) |
|---|---|
| Age (years) | 28.07 ± 7.28 |
| Age group band (years) | |
| 18–30 | 31 (67.4%) |
| 31–40 | 11 (23.9%) |
| 41–50 | 4 (8.7%) |
| Gender | |
| Male | 26 (56.5%) |
| Female | 20 (43.5%) |
| Total duration of illness (years) | 4.64 ± 3.44 |
| Age of onset (years) | 23.48 ± 6.76 |
| Site of onset | |
| Axilla | 28 (60.9%) |
| Groin | 10 (21.7%) |
| Chest | 3 (6.5%) |
| Breast | 1 (2.2%) |
| Back | 1 (2.2%) |
| Intermammary | 1 (2.2%) |
| Mons pubis | 1 (2.2%) |
| Perianal | 1 (2.2%) |
| Positive history of acne | 35 (76.1%) |
| Menstrual flare of disease | 11 (55%) |
| History of prior systemic treatment | 29 (63.0%) |
| Family history of HS | 6 (13.0%) |
| Positive history of alcohol intake | 13 (28.3%) |
| Age of starting alcohol intake (years) | 22.50 ± 5.13 |
| Total duration of alcohol intake (years) | 8.25 ± 5.01 |
| Positive history of smoking | 10 (21.7%) |
| Age of starting smoking (years) | 21.90 ± 4.93 |
| Total duration of smoking (years) | 9.80 ± 4.80 |
| Number of sites involved | 3.02 ± 1.20 |
| BMI (kg/m2) | 26.82 ± 4.90 |
SD: Standard deviation; HS: Hidradenitis suppurativa; BMI: Body mass index.
On clinical examination, 32.6% (n=15) of the patients had Hurley stage I disease [Figures 1a–1d]; 56.5% (n=26) of the patients had Hurley stage II [Figures 2a–2e], and 10.9% (n=5) had Hurley stage III disease.
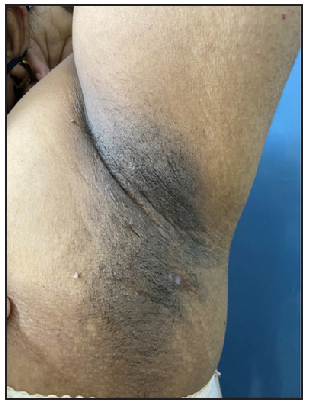
- 25-year-old female patient of HS with clinical Hurley stage I.
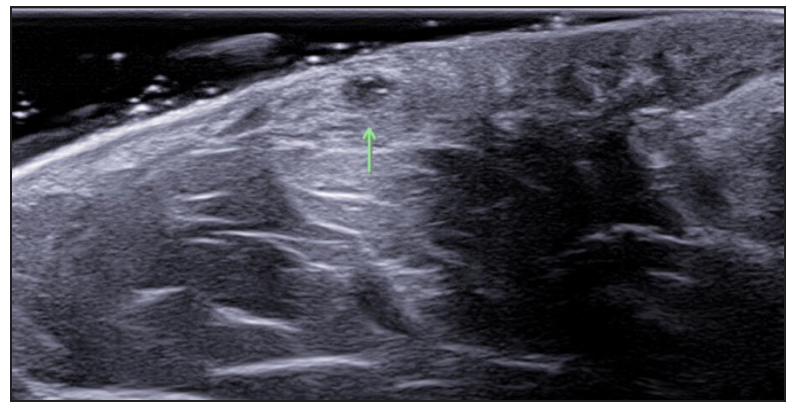
- Multiple fluid collections (green arrow), SOS-HS stage II.
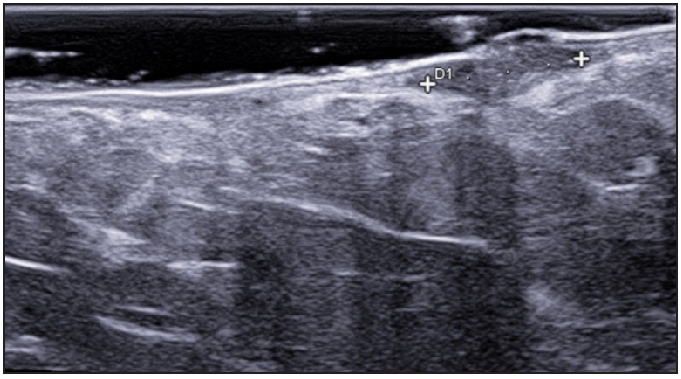
- Hypodermal hyperechogenic oedema of fistulous tracts.
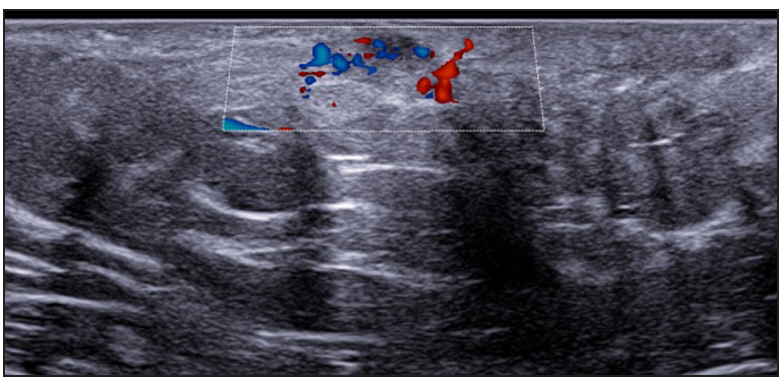
- Moderate vascularisation at periphery of fistulous tracts on colour doppler (Dotted rectangle represents the area with vascularity within the active site of involvement).
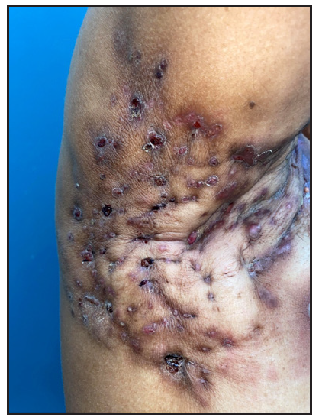
- A 52-year-old female patient of HS with clinical Hurley stage II.
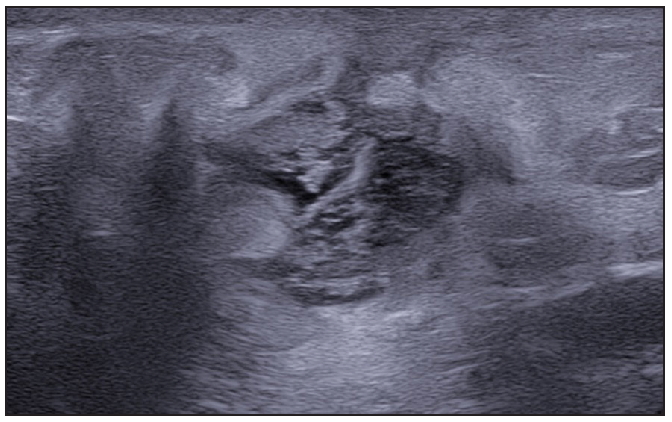
- Multiple fluid collections invading the fistulous tract and hypodermal hyperechogenic oedema.
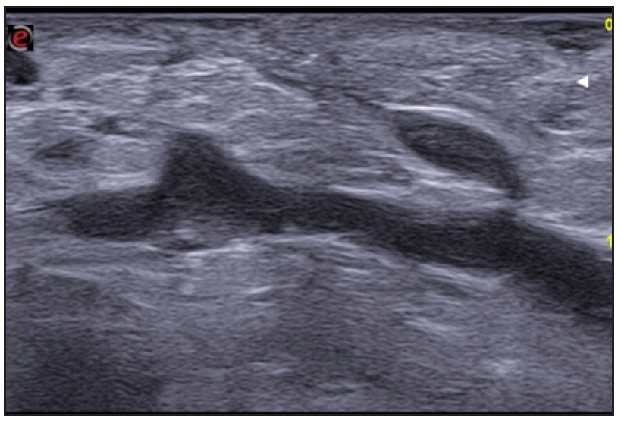
- Fistulous tracts in SOS-HS Stage III; with fibrotic scarring invading the fistulous tract and hypodermal hyperechogenic oedema. (SOS-HS: Sonographic staging of hidradenitis suppurativa.)
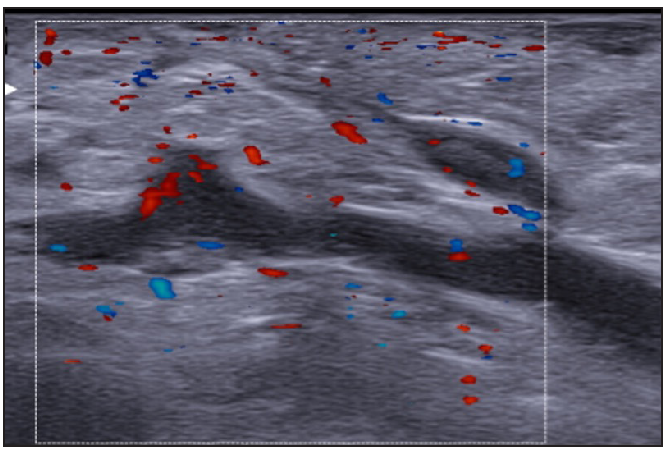
- Moderate vascularisation at the periphery and internal of fistulous tracts on colour doppler (Dotted rectangle represents the area with vascularity within the active site of involvement).
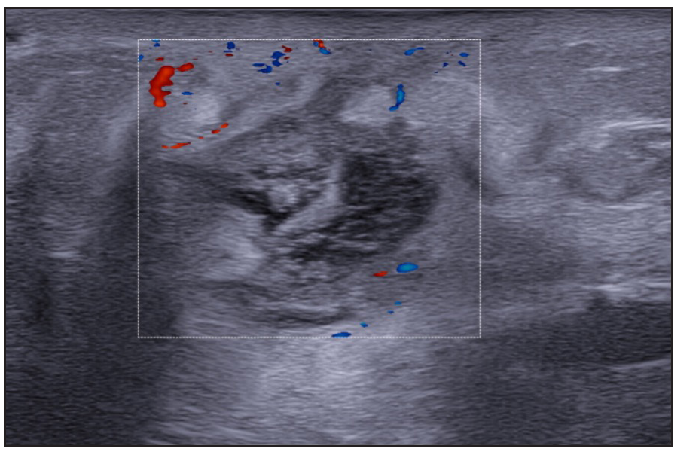
- Moderate vascularisation at the periphery and internal of fistulous tracts on colour doppler (Dotted rectangle represents the area with vascularity within the active site of involvement).
Among patients with clinically Hurley stage I disease, 46.7% (n=7) had stage I SOS-HS, 40% (n=6) had stage II SOS-HS and 13.3% (n=2) had stage III SOS-HS. Similarly, 26 patients had Hurley stage II disease, of whom 38.5% (n=10) patients had stage II SOS-HS and 61.5% (n=16) had stage III SOS-HS, All five patients with Hurley stage III disease also had stage III SOS-HS. Overall, 58.5% (n=24) patients had more severe disease (higher staging) by SOS-HS as opposed to their clinical Hurley stages (p<0.001) [Figure 3].
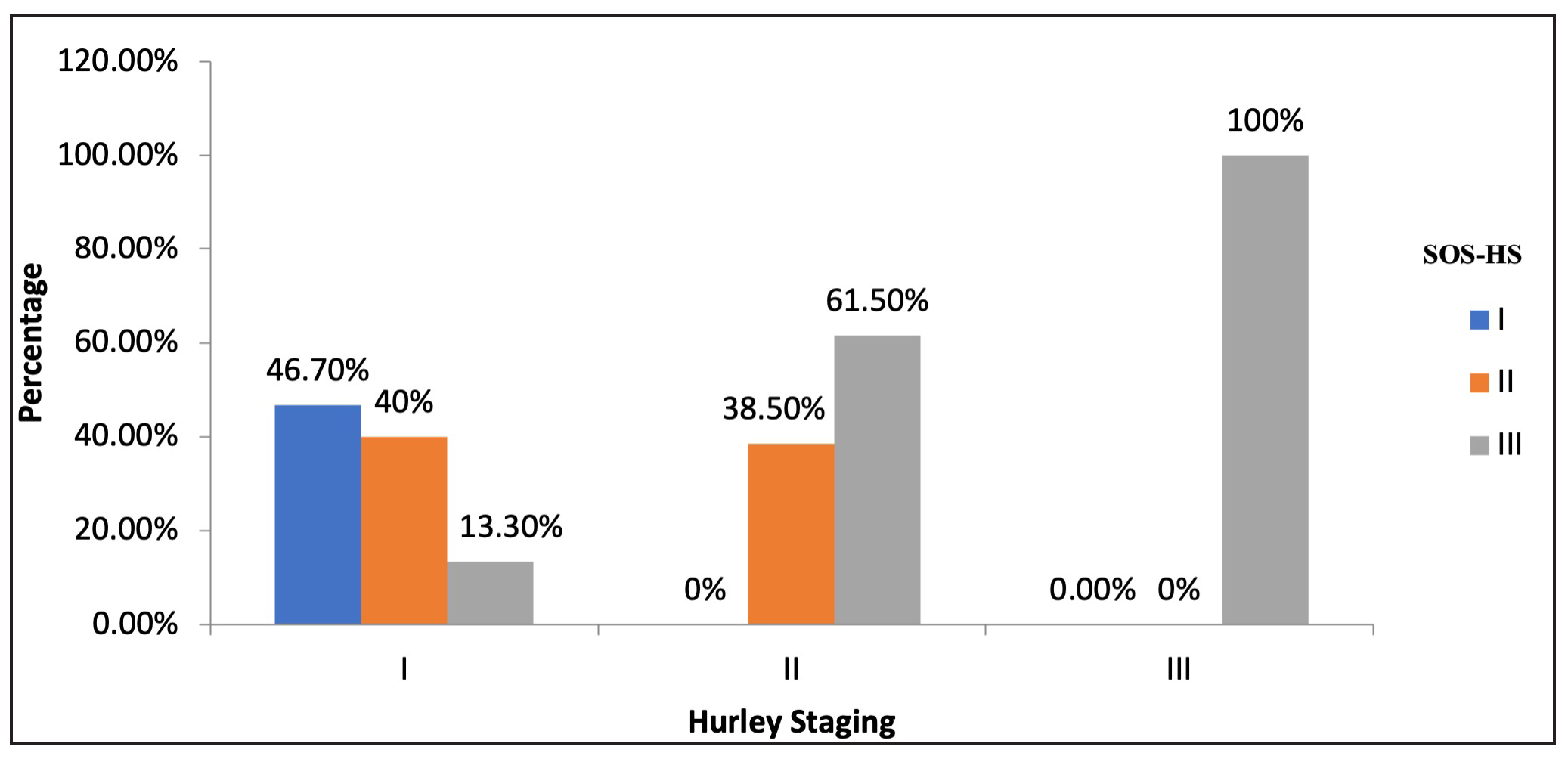
- Correlation between Hurley staging and SOS-HS. SOS-HS: Sonographic staging of hidradenitis suppurativa.
On comparing clinical staging with degree of vascularity on colour doppler, among Hurley stage I patients, 20% (n=3) had an absence of vascularisation, 46.7% (n=7) had minimal vascularisation and 33% (n=5) had moderate vascularisation. Similarly, among Hurley stage II patients, 26.9% (n=7), 50% (n=13), and 23.1% (n=6) were noted to have minimal, moderate and high vascularisation, respectively. In Hurley stage III patients, moderate and high vascularisation were noted in 20% (n=1) and 80% (n=4) patients, respectively. Overall, 26.8% (n=11) of patients demonstrated greater severity of vascularisation on colour doppler as compared to their clinical Hurley stages (p<0.002) [Figure 4].
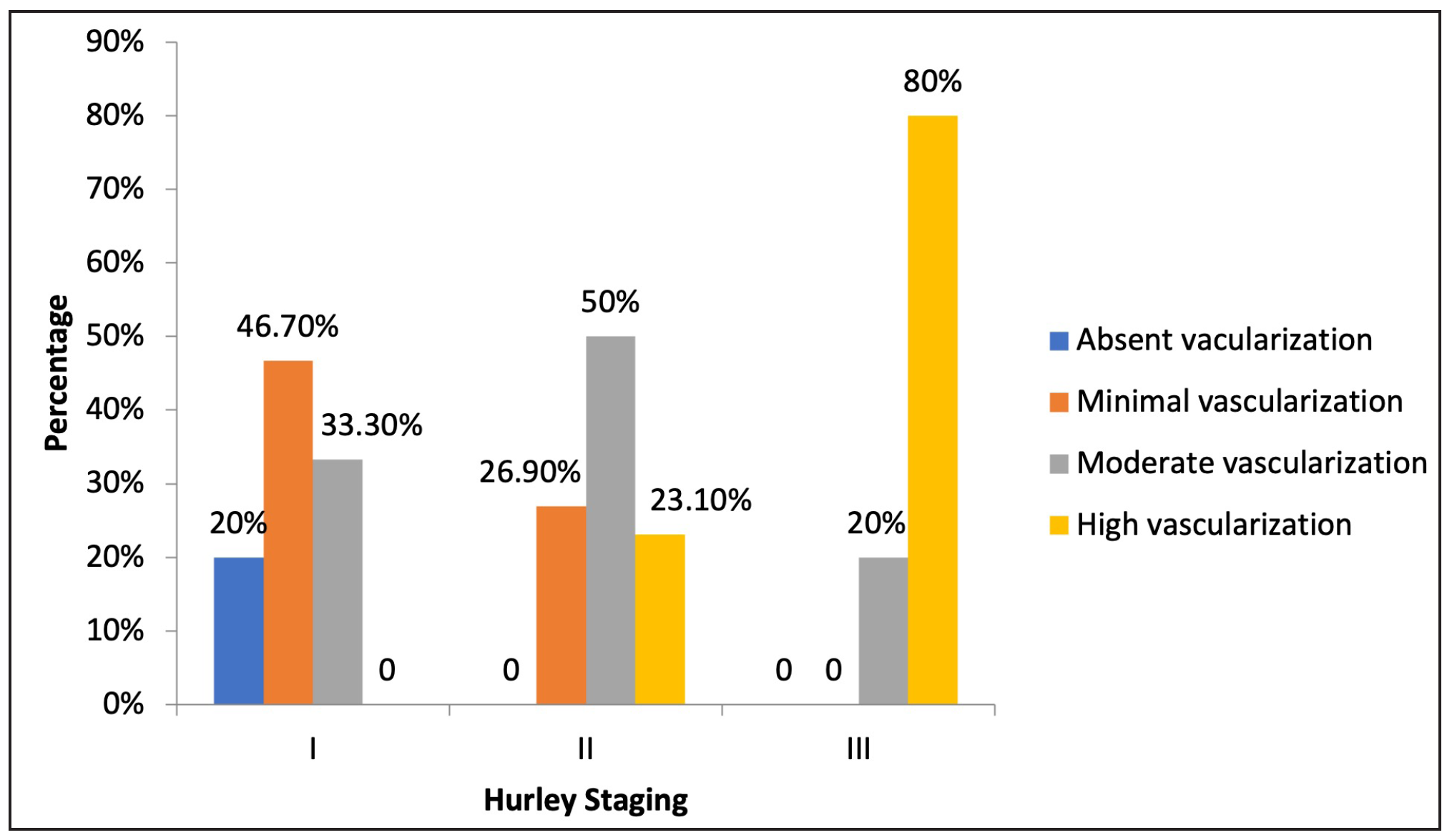
- Correlation between Hurley staging and vascular degree on color doppler
A significant association was observed between Hurley staging and degree of oedema of fistulous tracts on USG (p<0.006) [Supplementary Figure 1] and between Hurley staging and degree of fibrotic scarring of fistulous tracts on USG (p<0.002) [Supplementary Figure 2]. No correlation was observed between Hurley staging and type of fistulous tracts on USG [type 1=low fibrotic scarring with high or low oedema; type 2= high fibrotic scarring with low oedema; type 3= high fibrotic scarring with high oedema] (p=0.076), as well as pattern of vascularity of fistulous tracts (peripheral, internal or mixed) on colour doppler (p=0.142).’
Discussion
The use of ultrasonography for diagnostic and monitoring purposes in dermatology is not new. It has previously been used in a variety of skin tumours and other inflammatory disorders.11 High-resolution USG is an innovative tool for diagnostic characterisation and staging in HS and can deeply impact the management of this disease. It helps in mapping and identifying the extent of cutaneous lesions more precisely than physical examination. The use of the colour doppler helps in identifying the inflammatory activity of these lesions by measurement of the degree of vascularisation.
In this study, the majority of patients were males (56.5%; N=26). The mean patient age was 28.07 ± 7.28 years, the mean disease duration was 4.64 ± 3.44 years, and the body mass index of 26.82 ± 4.90. Male preponderance in HS has been documented in previous studies as well.12 About 50% of the patients were overweight (43.5%; N=25) which is a well-documented observation in previous studies. Active smoking and alcohol intake were observed in 21.7% (n=10) and 28.3% (n=13) patients, respectively, both of which are known risk factors for HS.12,13
The most commonly affected areas were the axilla in 28 (60.9%) and groin in 10 (21.7%) patients. In the study by Jemec et al., the groin was the most common region to be affected.14 In our study, a history of acne was present in 35 (76.1%) subjects, and 6 (13.1%) had one or more first-degree relatives with HS. The majority of patients in this study cohort were in Hurley stages 1 (32.6%; N=15) and 2 (56.5%; N=26). These findings are in concordance with the study by Zouboulis et al. where the majority of patients (68%) were in Hurley stage I,2 while in a study conducted by Kamat et al., the majority presented with Hurley stages 2 and 3.13
On correlating Hurley staging with USG, 24 (58.5%) patients had more severe disease (higher staging) by SOS-HS as compared to the Hurley Staging system (p<0.001). In previous studies performed by Napolitano et al., Martorell et al. and Nazzaro et al. in Europe, 28.7%, 44.7% and 35% of the patients, respectively, were found to have more severe HS as measured by ultrasonography SOS-HS when compared to the clinical Hurley staging system.5,8,15 In addition, Wortsman et al. found that USG findings modified the disease management in 82% of adult patients with the disease, and management changed from medical to surgical in 24% of patients.3 In another study based in India by Gogate et al., based on the USG findings, the management of 26% of the patients was changed from medical to surgical intervention.16 USG demonstrated that three patients had been misdiagnosed and had folliculitis (8.6%) and Crohn’s disease (4.3%). This study however lacked reporting of correlation between the clinical and USG staging, did not comment on vascularisation and did not assess in detail the degree of fibrotic scarring and oedema of fistulous tracts.
As suggested by our study results, the degree of vascularisation of HS lesions is often underestimated by clinical examination. The high-resolution USG findings, such as the presence of inflammatory activity and vascularity, have been shown to correlate with treatment response in HS. Studies have demonstrated that patients with high vascularity on colour doppler are more likely to have poorer responses to medical therapy and may benefit from early surgical interventions. Therefore, high-resolution USG can aid in predicting treatment outcomes and guiding therapeutic decisions, thereby optimising patient care.
High-resolution USG was also done to assess the degree of fibrotic scarring and oedema of fistulous tracts. Fibrotic scarring was graded as 0, 1 and 2 in increasing order of severity based on its location (periphery or invading fistulous tracts).9 Similarly, oedema was also graded as 0, 1 and 2 in increasing order of severity based on the presence of hypodermal hyperechogenicity and anechoic fluid.9
A significant association was seen between clinical Hurley staging and fibrotic scarring of fistulous tracts on USG (p<0.002), and overall, 18 (43.9%) patients had more severe fibrotic scarring. A significant association was also seen between clinical Hurley staging and oedema of fistulous tracts on USG (p<0.006). A more severe oedema was observed in 28 (68.2%) patients in USG. In a study by Wortsman et al., fibrotic scarring and oedema were present in 98% of patients. Also, 40% of cases had type 1, 25% had type 2 and 35% patients had type 3 fistulous tracts. The sonographic scores of these cases were SOS-HS II in 60% of patients and SOS-HS III in 40% which is consistent with the findings of our study.9
Colour doppler of the involved lesions was done to assess the degree of vascularity based on flow and pattern of vascularity (peripheral, internal, mixed). On comparing clinical Hurley staging with a degree of vascularisation on colour doppler, 11 (26.8%) patients had more severe vascularisation than expected (p<0.002). To the best of our knowledge, the correlation of colour doppler parameters with clinical Hurley staging has not been done before to assess the severity of disease in patients of HS. In a study by Caposiena Caro et al., 241 lesions were classified based on the degree and pattern of vascularity. They noted that the pattern of vascularity was peripheral in 143 lesions and mixed in 55 lesions, regardless of the clinical type. They also reported high vascularisation in 44 lesions, moderate in 79 lesions and minimal in 75 lesions; however, this study lacked a clinical correlation, an additional parameter studied in our cohort.10
No significant association was observed between Hurley staging and type of fistulous tracts on USG and pattern of vascularity on colour doppler. Hurley clinical staging alone provides insufficient information on the underlying processes that occur in HS. This fact is also validated by our study further as most HS patients, classified under Hurley I or II, were found to have a higher stage on USG SOS-HS. Furthermore, high-resolution USG and colour doppler revealed more severe morphological changes and a high degree of vascularity with mixed patterns which could not be accurately predicted from clinical findings. A higher degree of vascularisation can also have a clinical implication viz. of opting for medical therapy initially while devising treatment to control the inflammation, followed by surgical therapies.
High-resolution USG and colour doppler help to monitor treatment response by non-invasive measurement of the reduction in size or disappearance of lesions and decrease or lack of signal on colour doppler. Surgical intervention plays a crucial role in the management of HS, particularly in advanced cases refractory to medical therapy. High-resolution USG assists in surgical planning by delineating the extent of the disease, identifying the presence of sinus tracts and assessing the involvement of adjacent structures such as muscles and fascia. This information is invaluable for guiding the surgical approach, for minimising the risk of recurrence and optimising cosmetic outcomes. Its diagnostic utility, ability to stage the disease, guidance for surgical planning, and prediction of treatment response make high-resolution USG an indispensable component of the multidisciplinary approach to HS care. As our understanding of the pathophysiology of this condition continues to evolve, high-resolution USG is likely to play an increasingly prominent role in optimising patient outcomes and improving the quality of life for individuals living with this debilitating condition.
Limitations
Limitations of our study include a small sample size and cross-sectional design. The Hurley score is more consistent with the assessment of the damage caused by the disease than its evolution, being static to assess treatment response. The International HS Severity Score System (IHS4) score that dynamically validates HS severity and guides medical management was not used in this study. Ultrasonography is unable to detect lesions that measure ≤0.1 mm or those lesions with only epidermal location. In patients with HS however, this seldom happens because of the usual involvement of the dermis and hypodermis, with anatomic lesions of relevant size for high-resolution USG. Data regarding the change of treatment when a higher grade was diagnosed on USG was not recorded.
Conclusion
Owing to the growing interest and extensive research to assess disease severity and treatment response in HS, this study was undertaken to evaluate the role of high-resolution USG and colour doppler severity staging in patients with this condition. A higher number of patients were reported to have more severe staging on SOS-HS, higher vascularity of lesions on colour doppler and severe grading of fibrous scarring and oedema in comparison to the clinical Hurley staging. This suggests that the majority of patients are under-staged and so may be potentially under-treated based on clinical evaluation alone. The non-invasive nature, ability to visualise subcutaneous structures and dynamic assessment of blood flow make high-resolution USG and colour doppler a valuable addition to the diagnostic armamentarium in HS. Future research (using the IHS4 score) should focus on validating the utility of high-resolution USG in larger prospective studies and exploring its role in guiding novel treatment modalities, such as biologic agents and targeted therapies.
Ethical approval
This study was approved by the Institutional Review Board at PGIMER, Institutional Ethics Committee, number NK/7838/MD/362, dated September 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)–assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)–assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
References
- Ultrasound aids in diagnosis and severity assessment of hidradenitis suppurativa. Br J Dermatol. 2010;162:1400-2.
- [CrossRef] [PubMed] [Google Scholar]
- European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619-44.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound in-depth characterization and staging of hidradenitis suppurativa. Dermatol Surg. 2013;39:1835-42.
- [CrossRef] [PubMed] [Google Scholar]
- Color doppler as a tool for correlating vascularization and pain in hidradenitis suppurativa lesions. Skin Res Technol. 2019;25:830-4.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of clinical and ultrasound scores in patients with hidradenitis suppurativa: Results from an Italian ultrasound working group. J Eur Acad Dermatol Venereol. 2019;33:e84-e7.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical, biochemical, and ultrasonographic characteristics of patients with hidradenitis suppurativa in Northern Peninsular Malaysia: A multicenter study. Int J Dermatol. 2018;57:1454-63.
- [CrossRef] [PubMed] [Google Scholar]
- Color doppler ultrasound: A standard of care in hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2020;34:e616-e7.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound as a diagnostic and management tool in hidradenitis suppurativa patients: A multicentre study. J Eur Acad Dermatol Venereol. 2019;33:2137-42.
- [CrossRef] [PubMed] [Google Scholar]
- Color doppler ultrasound assessment of morphology and types of fistulous tracts in hidradenitis suppurativa (HS) J Am Acad Dermatol. 2016;75:760-7.
- [CrossRef] [PubMed] [Google Scholar]
- Power Doppler ultrasound assessment of vascularization in hidradenitis suppurativa lesions. J Eur Acad Dermatol Venereol. 2018;32:1360-7.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasonography in diagnostic dermatology: A primer for clinicians. Arch Dermatol Res. 2023;315:1-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Hidradenitis suppurativa: Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-58.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-epidemiological characteristics of hidradenitiss: A retrospective cohort study from a tertiary care centre in Northern India. Indian Dermatol Online J. 2021;12:561-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical practice. Hidradenitis suppurativa. N Engl J Med. 2012;366:158-64.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of clinical and sonographic scores in a cohort of 140 patients with hidradenitis suppurativa from an Italian referral centre: A retrospective observational study. Eur J Dermatol. 2018;28:845-7.
- [CrossRef] [PubMed] [Google Scholar]
- A pilot real-world study of ultrasonography findings of hidradenitis suppurativa in Indian patients and its diagnostic and therapeutic implications. Indian J Radiol Imaging 2024.. 2024;34(4):603-611.
- [Google Scholar]







