Translate this page into:
Successful use of oral sirolimus – A mammalian target of rapamycin (m-TOR) inhibitor in the treatment of kaposiform haemangioendothelioma with Kasabach-Merritt phenomenon
Corresponding author: Dr. Senkadhir Vendhan, Department of Dermatology, Southern Railways Headquarters Hospital, Perambur, Chennai, India. vendhan100@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Neema S, Vendhan S, Vasudevan B, Priya S, Kashif AW, Pandey S. Successful use of oral sirolimus – A mammalian target of rapamycin (m-TOR) inhibitor in the treatment of kaposiform haemangioendothelioma with Kasabach-Merritt phenomenon. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_871_2024
Dear Editor,
A two-year-old girl child, born to non-consanguineous parents without a family history of similar illnesses, presented with a swelling on her left thigh since birth. The initial swelling, measuring approximately 3 × 3 cm, gradually extended to involve her lower abdomen, gluteal region and more than two-thirds of her left thigh. Prior to coming to our center, she had received multiple courses of oral steroids with partial relief. On examination, the child appeared irritable with tenderness over the affected area and mooning of the face. There was an erythematous, warm, tender nodulo-plaque involving the left vulva, gluteal region and thigh, measuring 42 × 30 cm, with diffuse swelling in the affected regions that responded well to oral sirolimus [Figure 1a–1f]. Petechiae were evident across her body and pain limited movement around her left hip. Investigations revealed haemoglobin of 5.5 g/dL, total leukocyte count of 13,100/mm3 and platelets of 40,000/mm3. Fibrinogen levels were 60 mg/dL and D-dimer values exceeded 10,000 ng/mL. Contrast-enhanced magnetic resonance imaging indicated soft tissue lesions causing thickening and involving the muscles of the abdominal wall, retroperitoneum, gluteal region and left thigh [Figure 2a and 2b]. Histopathological analysis from a skin biopsy showed a vaso-formative lesion extending from the reticular dermis into the subcutaneous tissue and muscle fibres. It revealed numerous ectatic, congested, non-communicating vascular channels with a flattened endothelial lining, surrounded by dense collagen and areas containing spindle to fibroblast-like cells. Immunohistochemistry studies confirmed the diagnosis with positive CD34 and D2-40 staining highlighting the ectatic vascular lumens [Figure 3a–3d]. The patient was diagnosed with Kasabach-Merritt syndrome associated with kaposiform haemangioendothelioma. Treatment included transfusion of two units of packed red blood cells and fresh frozen plasma, along with oral prednisolone 7.5 mg once daily and sirolimus 0.5 mg twice daily (adjusted to 0.8 mg/m2). Steroids were gradually tapered and discontinued over six months. Growth and bone density were assessed to look for steroid-related side effects and were found to be normal. Significant improvement was observed after one year of treatment, with nearly 90% resolution of lesions and improvement of haematological parameters (haemoglobin 8.5 g/dL, total leukocyte counts 8300/mm3, platelets 120,000/mm3 and normal fibrinogen and D-dimer levels). Sirolimus dosage was reduced to 0.5 mg once daily, and the patient tolerated the therapy well without adverse effects.
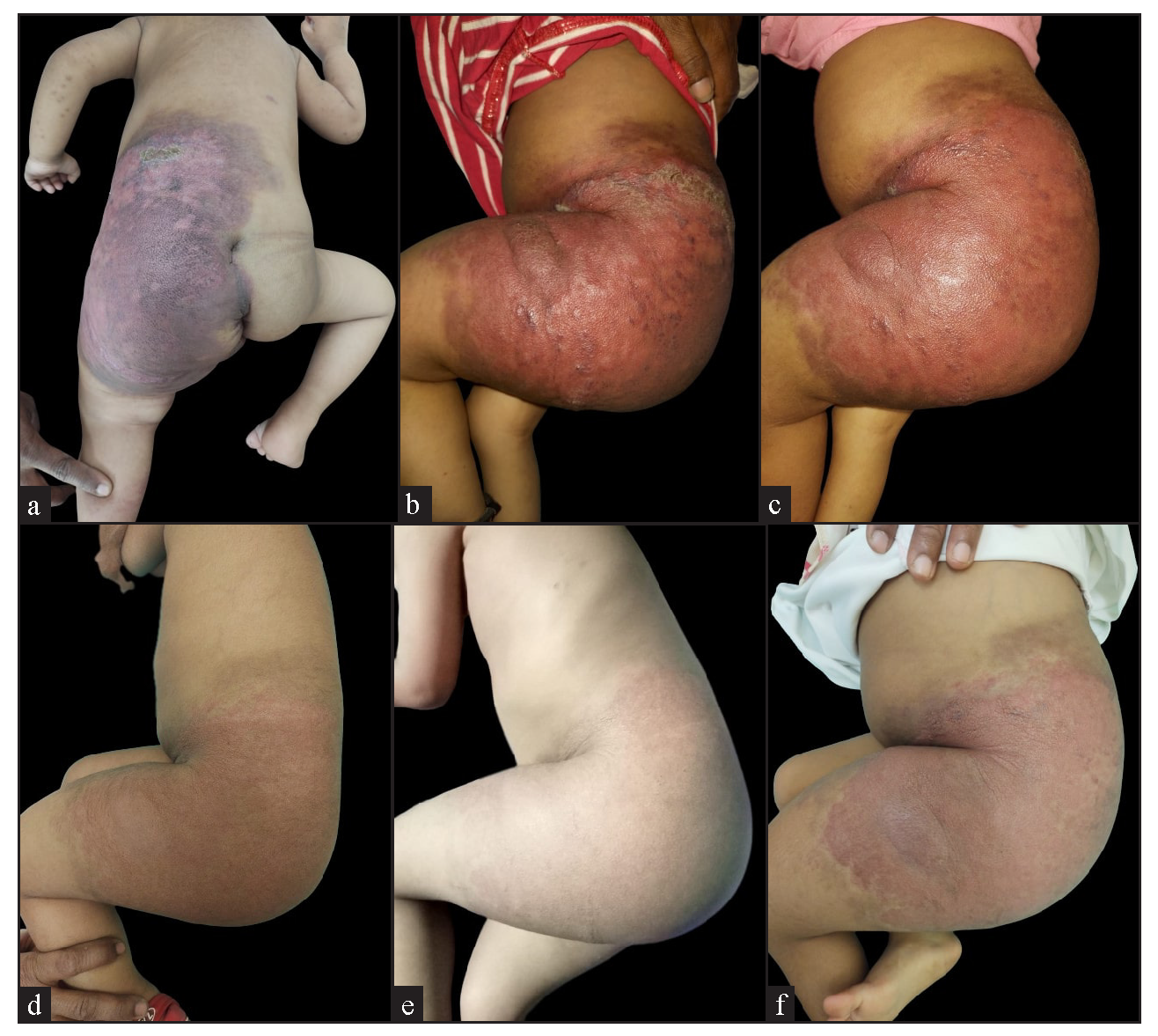
- Clinical images of a child showing a well-defined erythematous nodulo-plaque with diffuse swelling over the abdomen, gluteal region, and left thigh, including (a) baseline front and (b) lateral views, (c-e) progressive changes during follow-up, and (f) the clinical images after six months of treatment.
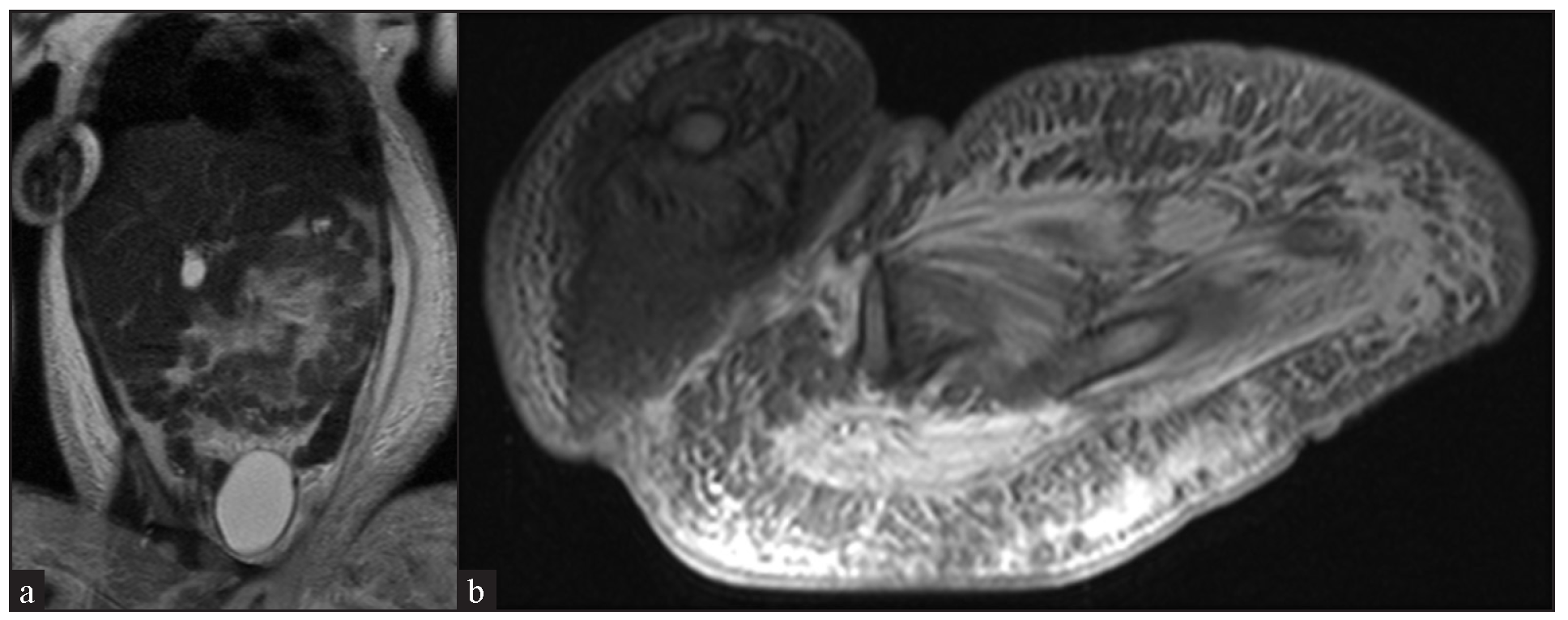
- Contrast-enhanced magnetic resonance imaging of abdomen and thigh showing enhanced soft tissue swelling and possible veno-lymphatic malformation in T2 (a) Coronal section and (b) axial section.
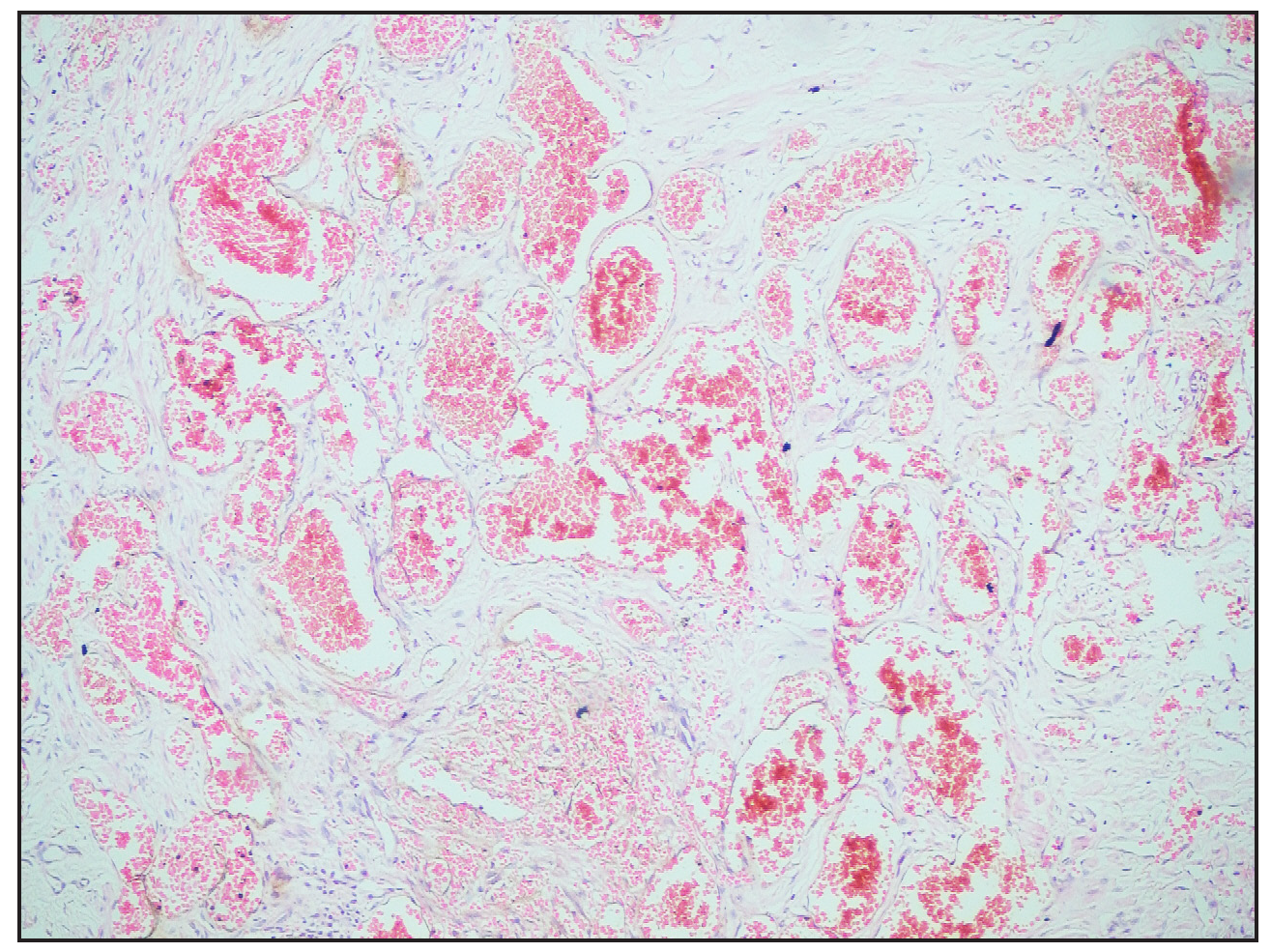
- Histopathological image showing numerous ectatic and congested vascular channels (Haematoxylin and eosin, 10×).
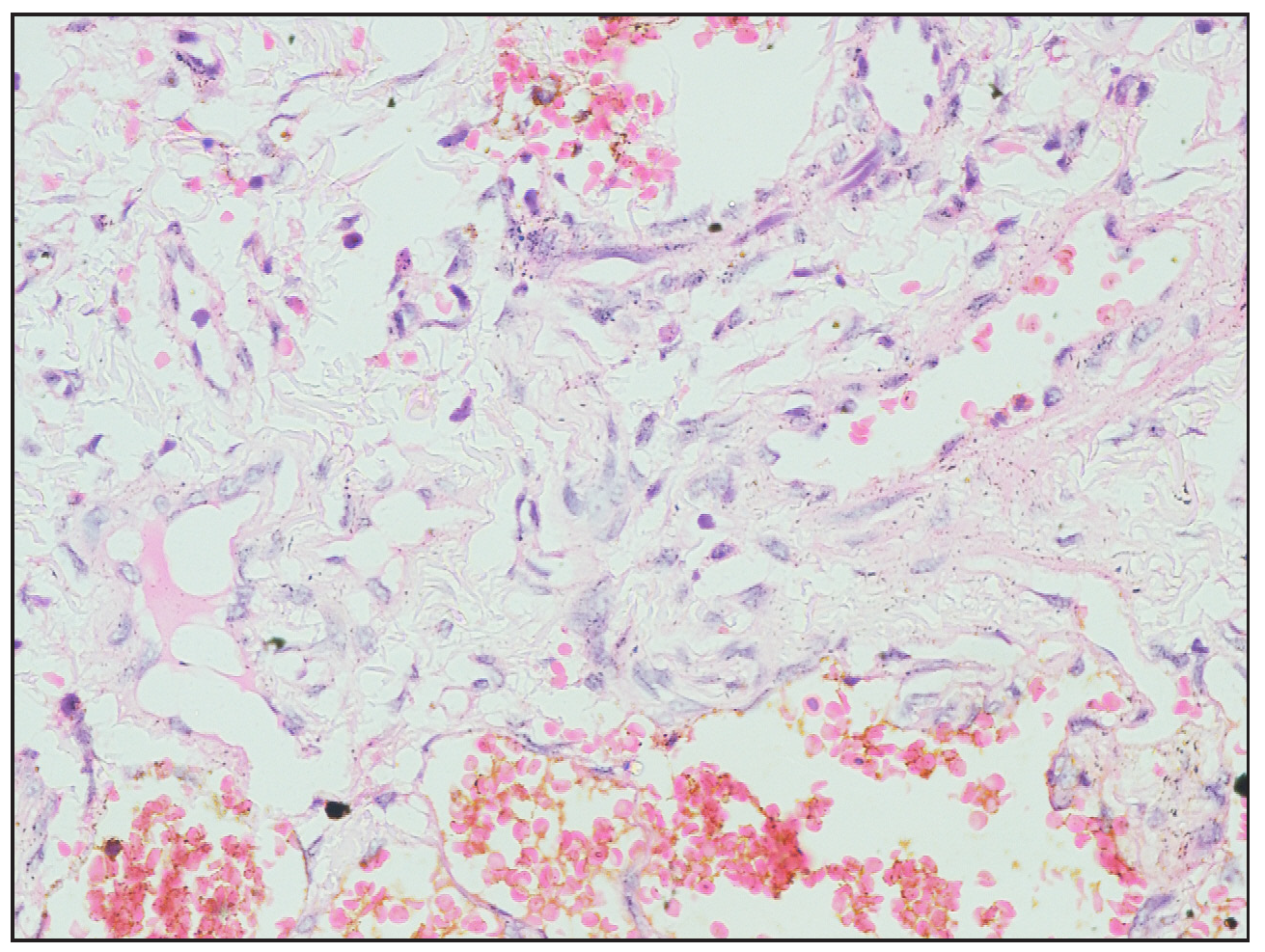
- Histopathological images showing flattened endothelial lining and spindle to fibroblast-like cells (Haematoxylin and eosin, 40×).
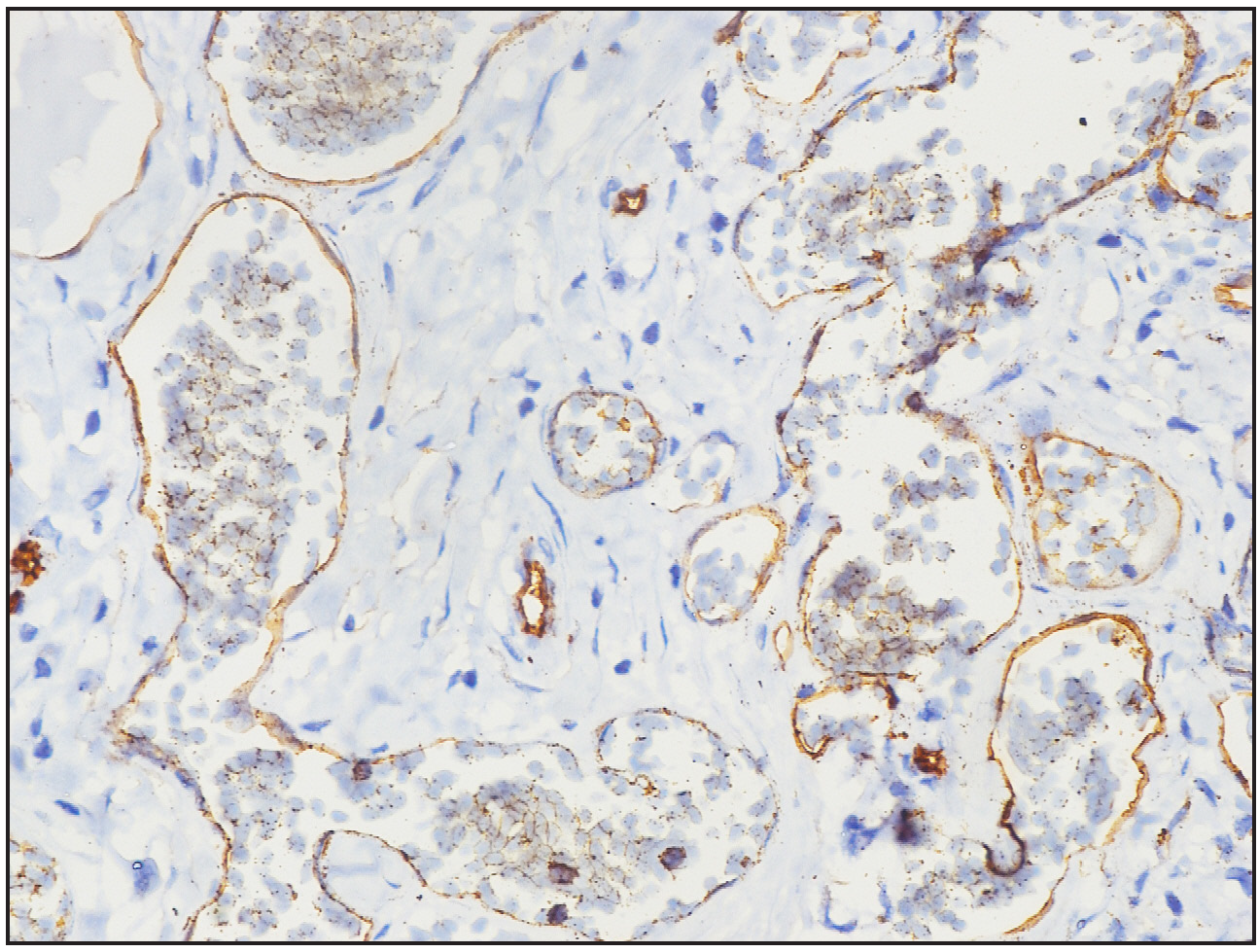
- Immunohistochemistry image showing CD34 highlighting the ectatic vascular lumens (40× magnification).
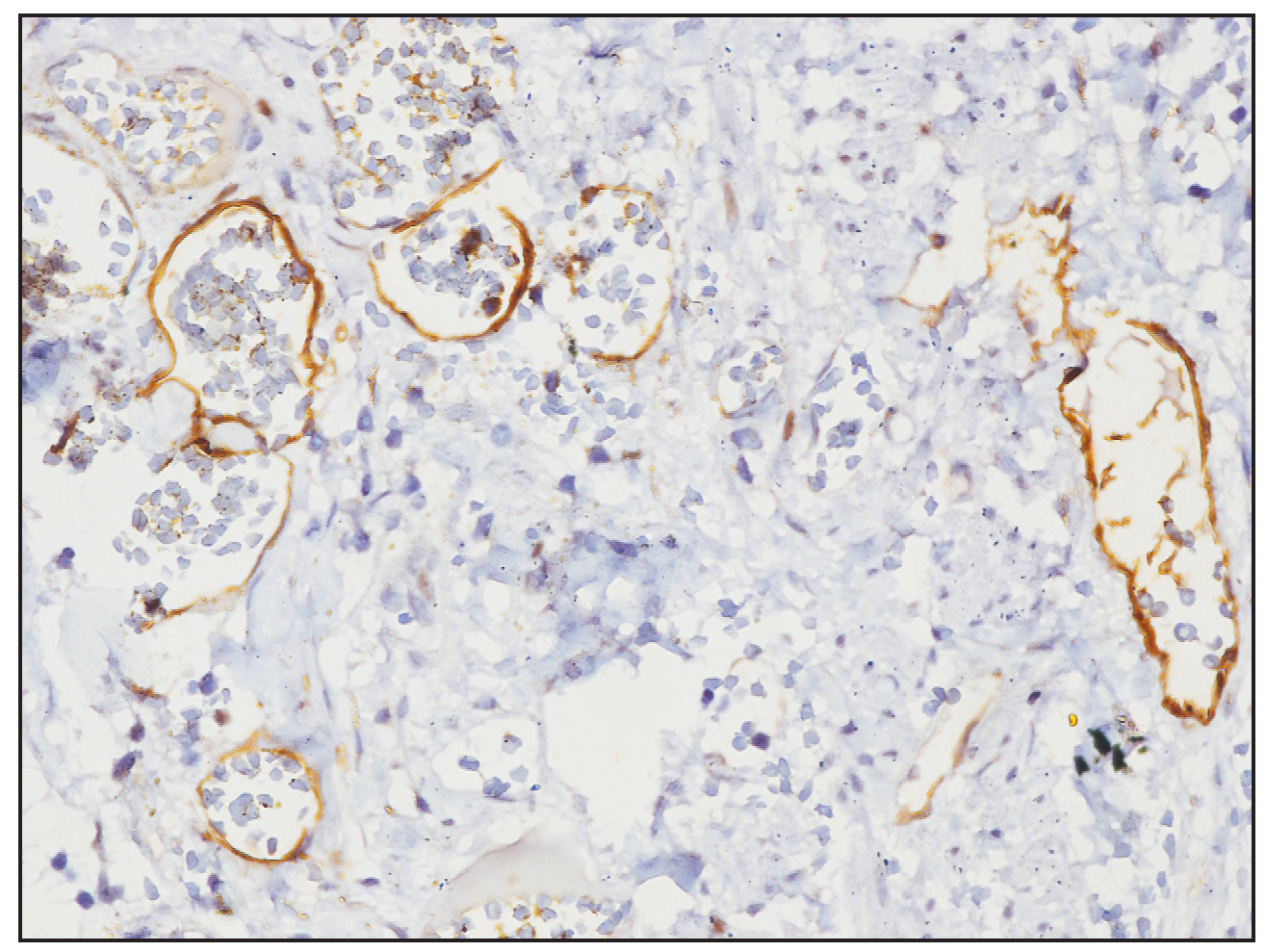
- Immunohistochemistry image showing D2-40 highlighting the ectatic vascular lumens (40× magnification).
Kaposiform haemangioendothelioma is a rare and aggressive vascular tumour primarily seen in infants, with an incidence of 0.071 per 100,000 per year, often appearing in the first year of life.1 It is frequently associated with the Kasabach-Merritt phenomenon, a severe complication involving thrombocytopaenia and coagulopathy due to platelet trapping and coagulation activation within the lesion.2 Histologically, kaposiform haemangioendothelioma shows lobulated nodules with slit-like vessels lined by spindle-shaped endothelial cells, often containing erythrocytes, platelet thrombi, hyaline bodies and haemosiderin deposits.3
Treatment options for kaposiform haemangioendothelioma include corticosteroids, vincristine, sirolimus, ticlopidine, aspirin, interferon-alpha and propranolol. Sirolimus, in combination with short-term systemic corticosteroids, is recommended as first-line therapy for severe Kasabach-Merritt phenomenon.4 Sirolimus is administered at a dose of 0.8 mg/m2 twice daily, with adjustments to maintain therapeutic levels (8–15 ng/mL). Commonly reported adverse effects include mucositis, hyperlipidaemia and increased susceptibility to infections, necessitating close monitoring of drug levels, lipid profile and infection markers. Other potential side effects include thrombocytopaenia, anaemia and delayed wound healing, which require dose adjustments or temporary discontinuation if severe. Despite these adverse effects, sirolimus is generally well tolerated when therapeutic drug levels are maintained. The optimal duration of sirolimus therapy is variable, typically ranging from 12 to 24 months, depending on the patient’s response to treatment. Maintenance therapy is often required to prevent relapse, especially in cases with the Kasabach-Merritt phenomenon, and gradual tapering of sirolimus is recommended once significant improvement is observed. Long-term use at lower maintenance doses is generally associated with fewer adverse effects, making it a suitable option for extended treatment periods in certain cases. While more than 300 reports have documented the successful use of sirolimus in the treatment of kaposiform haemangioendothelioma, particularly in cases complicated by the Kasabach-Merritt phenomenon, there is still a need for standardised treatment protocols regarding optimal therapy duration and maintenance strategies.5,6 While surgical resection is considered for localised tumours amenable to complete removal, it is generally avoided in cases where it could exacerbate coagulopathy.7 In our patient’s case, a reduction in tumour size was achieved with oral prednisolone and sirolimus, without adverse effects.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Kaposiform Hemangioendothelioma with Kasabach-Merritt Phenomenon. Indian J Pediatr. 2021;88:1142-4.
- [CrossRef] [PubMed] [Google Scholar]
- Kaposiform haemangioendothelioma: Clinical features, complications and risk factors for Kasabach-Merritt phenomenon. Br J Dermatol. 2018;179:457-63.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Kaposiform haemangioendothelioma: A review with emphasis on histological differential diagnosis. Pathology. 2017;49:356-62.
- [CrossRef] [PubMed] [Google Scholar]
- Sirolimus as initial therapy for kaposiform hemangioendothelioma and tufted angioma. Pediatr Dermatol. 2018;35:635-8.
- [CrossRef] [PubMed] [Google Scholar]
- Effective low-dose sirolimus regimen for kaposiform haemangioendothelioma with Kasabach-Merritt phenomenon in young infants. Br J Clin Pharmacol. 2022;88:2769-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sirolimus plus prednisolone vs sirolimus monotherapy for kaposiform hemangioendothelioma: A randomized clinical trial. Blood. 2022;139:1619-30.
- [CrossRef] [PubMed] [Google Scholar]
- Refractory Kasabach-Merritt phenomenon successfully treated with sirolimus, and a mini-review of the published work. J Dermatol. 2015;42:401-4.
- [CrossRef] [PubMed] [Google Scholar]





